A Raman spectroscopic study of uranyl minerals from Cornwall, UK†
Abstract
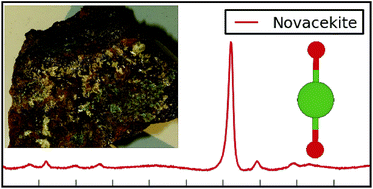
In the fields of nuclear forensics, geology and environmental science, it is important to be able to rapidly identify an unknown sample of uranyl mineral. Raman spectroscopy provides a fast, non-destructive and portable strategy for collecting data, which can then be compared against a set of known experimental information. We present a Raman study of a selection of uranyl minerals from Cornwall, UK. This includes the first Raman spectrum published for the uranyl arsenate mineral, nová![[c with combining breve]](https://www.rsc.org/images/entities/char_0063_0306.gif) ekite. These spectra were collected under a standard set of conditions, using three excitation wavelengths, 325, 532 and 785 nm, the latter typically providing spectra with little fluorescence and the best resolution. The vibrational properties of these minerals are characterised by the symmetric stretching mode of the uranyl cation, seen between 750–900 cm−1, though the exact position varies with respect to the local environment. To discriminate between samples, the rest of the spectrum must be considered; the poly-anions in the structure provide a fingerprint set of Raman bands. An added complication occurs when samples of the same mineral from different mines demonstrate variations in their Raman spectra; this emphasises the need for data to be collected from a variety of locations, but also suggests that other experimental techniques could provide complementary information.
ekite. These spectra were collected under a standard set of conditions, using three excitation wavelengths, 325, 532 and 785 nm, the latter typically providing spectra with little fluorescence and the best resolution. The vibrational properties of these minerals are characterised by the symmetric stretching mode of the uranyl cation, seen between 750–900 cm−1, though the exact position varies with respect to the local environment. To discriminate between samples, the rest of the spectrum must be considered; the poly-anions in the structure provide a fingerprint set of Raman bands. An added complication occurs when samples of the same mineral from different mines demonstrate variations in their Raman spectra; this emphasises the need for data to be collected from a variety of locations, but also suggests that other experimental techniques could provide complementary information.

 Please wait while we load your content...
Please wait while we load your content...