Surface chemistry of PET for enhancing its antifouling properties
Maria Jesus Perez-Roldan†
,
Dominique Debarnot and
Fabienne Poncin-Epaillard*
LUNAM Université, UMR Université du Maine – CNRS no. 6283, Institut des Molécules et Matériaux du Mans – Département Polymères, Colloïdes et Interfaces, Avenue Olivier Messiaen, 72085 Le Mans Cedex, France. E-mail: fabienne.poncin-epaillard@univ-lemans.fr
First published on 19th November 2014
Abstract
In this work, highly hydrophilic PET surfaces were obtained by helium and oxygen plasma treatments. Plasma-treated samples were then grafted with PEG, Pluronic F68, Pluronic F108, mixed solutions of Pluronic and surfactant (nonaethylene glycol monodecyl ether, sodium taurodeoxycholate, hexadecyltrimethyl ammonium bromide). Grafted surfaces were characterized by X-ray photoelectron spectroscopy and contact angle measurements. Surface energy calculations showed a high affinity of hexadecyltrimethyl ammonium bromide to O2 plasma-treated surfaces. In relation to this type of surface chemistry, the anti-fouling character of such a modified PET surface was studied by confocal microscopy. Evidence of reduction of the Immunoglobulin G adhesion by around 70%, 60% and 50% for plasma-treated surfaces grafted with PEG, Pluronic F108 and Pluronic F68, respectively is given. A remarkable increase in the anti-fouling properties was also observed on aged grafted surfaces.
Introduction
Polymer materials able to reduce or prevent biofouling and biocontamination are of great interest in numerous sectors such as the food industry, biosensing applications or medical devices. Therefore, to achieve materials in which the interaction of biomolecules with the surface can be controlled, an appropriate surface modification is needed. Two opposite surface properties of a material may achieve such a goal, i.e. superhydrophilic or phobic character. The former leads to non-adhesion thanks to the formation of a water monolayer acting as a weak boundary layer avoiding any permanent adhesion of biotargets. Among the different techniques used for changing polymer surface properties,1–3 plasma modification has been broadly used due to its ability to alter the surface without modification of the bulk characteristics.4–9In this work, a polymer commonly used in biomedical applications, polyethylene terephthalate (PET) has been modified by means of helium and oxygen plasma treatments. Such treatments produce a chemical modification on the PET surface. Reactive gases like O2 can increase the oxygen-containing groups on the surface and produce an increment of the hydrophilic character of the surface.10 But also inert gases, like He, that produce a surface cross-linking, can increase the surface wettability,11,12 factor that has a determining influence on biological adsorption.13
Furthermore, through the plasma treatment reactive surfaces are produced that allow the grafting of polymer chains on the surface,14 therefore, specific functionalities can be provided to the surface and consequently the bioadhesive properties can be enhanced or reduced by selecting the proper coatings. Therefore, different polymers were grafted after the plasma treatment of PET, in order to confer to the surface antifouling properties. The polymer selected for the grafting was Pluronic, a well known antifouling copolymer.15 In particular, Pluronic F68 and Pluronic F108 were used, two amphiphilic triblock copolymer with similar EO/PO ratio but different length chains.
Besides, Pluronic solutions were mixed with surfactants showing different properties in order to evaluate and to control their effect on the surface energy. For that, nonaethylene glycol monodecyl ether (C12E9), sodium taurodeoxycholate (STDC) and hexadecyltrimethyl ammonium bromide (CTAB) were selected due to their nonionic (C12E9), anionic (STDC) and cationic (CTAB) character.
The chemical composition of grafted surfaces was characterized by X-ray photoelectron spectroscopy (XPS) and the surface free energy was studied by contact angle measurements.
Moreover, the adhesion properties conferred to the PET substrates by the grafting of these polymers were analyzed by confocal microscopy.
Since the most common approach to enhance the protein resistant of the surfaces is to immobilize poly(ethylene glycol) (PEG), plasma treated surfaces were grafted with PEG based coatings in order to compare the results obtained with a well known anti-bioadhesive polymer.16 Besides, an important factor in hydrophilic surfaces is the aging effect, i.e. hydrophobic recovery that changes the surface chemistry of the polymer over the storage period affecting the wettability and the adhesion properties of plasma-treated surfaces.17 Hence, the anti-fouling character of aged surfaces was studied.
Experimental
Chemical and materials
Poly(ethylene terephthalate) films (PET-G Veralite 200 – VPC Display) cut in pieces of approximately 2 × 2 cm2, were cleaned by ultrasonication in ethanol (5 min) and then rinsed with distillated water and dried under compressed air.Poly(ethylene glycol) (PEG), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol), so called Pluronic F68 (PF68, M = 8350 g mol−1, CMC at 25° = 0.04 mM) and Pluronic F108 (PF108, M = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, CMC at 25° = 0.02 mM), nonaethylene glycol monodecyl ether (C12E9, M = 583 g mol−1, CMC = 0.08 mM), sodium taurodeoxycholate (STDC, M = 522 g mol−1, CMC = 4 mM), hexadecyltrimethyl ammonium bromide (CTAB, M = 365 g mol−1, CMC = 1 mM) were purchased from Sigma Aldrich. The critical micelle concentrations (CMC) of these surfactants, provided by the supplier, were determined at 25° in aqueous solution. Surfactant solutions were prepared with distilled water by overnight stirring. Mixed solutions of surfactants were prepared at 1
600 g mol−1, CMC at 25° = 0.02 mM), nonaethylene glycol monodecyl ether (C12E9, M = 583 g mol−1, CMC = 0.08 mM), sodium taurodeoxycholate (STDC, M = 522 g mol−1, CMC = 4 mM), hexadecyltrimethyl ammonium bromide (CTAB, M = 365 g mol−1, CMC = 1 mM) were purchased from Sigma Aldrich. The critical micelle concentrations (CMC) of these surfactants, provided by the supplier, were determined at 25° in aqueous solution. Surfactant solutions were prepared with distilled water by overnight stirring. Mixed solutions of surfactants were prepared at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume and they were kept overnight stirring for stabilization.
1 in volume and they were kept overnight stirring for stabilization.
Immunoglobulin G from rabbit serum (IgG), anti-rabbit IgG FITC conjugate (Ab-IgG-FITC) developed in goat, egg albumin and phosphate buffered saline (PBS) were purchased from Sigma Aldrich. All protein solutions were prepared in PBS.
Plasma treatment
Experiments were performed in a home-made radiofrequency (RF) plasma reactor. The system was pumped with a turbomolecular pump with the nominal pumping speed of 900 m3 h−1 backed with a two-stage oil rotary pump with a pumping speed of 25 m3 h−1. The discharge chamber is made of stainless steel (30 × 15 × 10 cm3). In the upper part of the chamber a flat rectangular electrode is powered by a RF generator via a matching network that is in turn connected to a 13.56 MHz RF generator. Gases are leaked into the discharge chamber through a precise flow controller. Commercially available, highly purified helium (purity > 99%, Air Liquide) and oxygen were used in the experiments. Plasma treatments were carried out at a fixed power of 75 W and a flow rate of 10 sccm and 50 sccm for He and O2 respectively. The pressure of the chamber before gas injection was of 6.4 10−5 mbar. After injection of gases the pressure reached 3.3 10−4 mbar and 1.3 10−2 mbar for He and O2, respectively.Grafting procedure
Immediately after the plasma-treatment, PET surfaces were dipped for 3 min in surfactant solutions at room temperature. Then, the samples were extensively rinsed with distilled water to remove the unstably adsorbed molecules and then softly dried under dry compressed air. Since even a soxhlet extraction did not remove the polymeric layer, the final sample will be considered as grafted. Samples grafted for biocharacterization experiments were kept under the fume hood (1 h) for longer drying before protein immobilization procedure. The grafting process as a control was also run onto a native PET surface. However in such a case after the rinsing step, the surface analyses (XPS and wettability) give evidence of an only PET surface.Surface characterization
Contact angles (CA) were measured using a goniometer (Ramé-Hart 100-00) at room temperature. For each measurement, a 3 μl drop was placed on the surface. Surface free energy (γt) and both polar (γp) and dispersive (γd) components were obtained from the measurements with glycerol and ultra-pure water using the Fowkes formula:Meanwhile the acid (γ+) and basic (γ−) characters of the surfaces were obtained from the measurements with ultra-pure water, diiodomethane (Fluka, purity > 99%) and glycerol (Sigma Aldrich) applying the Oss Wood Chaudury approach:
Data for the used liquid probes are given: water: γt = 72.8 mJ m−2, γd = 21.8 mJ m−2, γp = 51.0 mJ m−2, γ+ = γ− = 25.5 mJ m−2; diiodomethane: γt = 50.8 mJ m−2, γd = 48.5 mJ m−2, γp = 2.3 mJ m−2, γ+ = γ− = 0.0 mJ m−2; glycerol: γt = 64.0 mJ m−2, γd = 34.0 mJ m−2, γp = 30.0 mJ m−2, γ+ = 3.92 mJ m−2, γ− = 57.4 mJ m−2.
For calculation; a drop from each liquid was measured on three different samples.
Mapping of contact angle distribution water drops on the treated surfaces was carried out using a picoliter dosing system DSA100M (Krüss GmbH, Hamburg). An 8 × 8 matrix of 5 × 5 μm2 area was designed using DSA Mapping Editor software (Krüss GmbH, Hamburg) to obtain a contact angle map of the surface. The CA value of every drop (64 drops in total) was measured by the DSA100M software (4 measurements per second). CA maps were taken 1 h after the grafting procedure was completed (plasma treatment, grafting, washing, drying). The average of the CA values was used to draw the map using the Matlab software.
X-ray photoelectron spectroscopy (XPS) measurements were performed with an AXIS NOVA Spectrometer (KRATOS Analytical, UK). The samples were irradiated with monochromatic AlKα X-rays (hν = 1486.6 eV) using X-ray spot size of 100 μm diameter and a take off angle of 90° with respect to the sample surface. The charge compensation system was used on all samples and all spectra were corrected by setting the C1s hydrocarbon component to 285.00 eV binding energy. For each sample, a survey spectrum (0–1350 eV) was recorded at pass energy of 160 eV. Measurements were performed on samples stored for a period of 10 days at laboratory conditions. Sample compositions were obtained from the survey spectra after Shirley background subtraction and using the RSF (Relative Sensitivity Factors) 0.78 for O and 0.278 for C. In addition one set of high-resolution spectra was recorded on each sample at pass energy of 20 eV. The data were processed using Casa-XPS v2.3.16 (Casa software, UK). The core level envelopes were fitted with Gaussian–Lorentian function (G/L = 30) and variable full width half maximum previous background subtraction.
Imaging of protein coated samples was performed by confocal microscopy, Leica TCS-SP2 (Leica Microsystems Heidelberg, Germany), working with FITC filter (488 nm), a beam intensity of 50% and the PMT1 set at 600 V for all the images. In order to compare the bioactivity of treated and non-treated surfaces, half area of every sample was covered with a mask during the plasma treatment to avoid the plasma exposure. In that way, half sample was plasma treated and half sample remained untreated. Samples were incubated in IgG (20 μg ml−1) at 4° overnight. After a generous washing with PBS to remove not well adhered proteins, samples were incubated with egg albumin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) at room temperature for 1 hour in static mode. Then, washed quite a lot with PBS and incubated with Ab-IgG-FITC (10 μg ml−1) at room temperature for 1 h in static mode. Finally samples were carefully cleaned with PBS. For each treatment, a total of three samples were analyzed. From each sample, five confocal images were taken from plasma treated and non treated areas and the mean intensity of the five images was averaged. Then, the average intensity from treated surfaces was divided by the average intensity of the non plasma treated areas to obtain their intensity ratio.
100) at room temperature for 1 hour in static mode. Then, washed quite a lot with PBS and incubated with Ab-IgG-FITC (10 μg ml−1) at room temperature for 1 h in static mode. Finally samples were carefully cleaned with PBS. For each treatment, a total of three samples were analyzed. From each sample, five confocal images were taken from plasma treated and non treated areas and the mean intensity of the five images was averaged. Then, the average intensity from treated surfaces was divided by the average intensity of the non plasma treated areas to obtain their intensity ratio.
Results and discussion
The adhesion of micro-organisms can be affected by the physico-chemical properties of substrate, such as its hydrophilic or hydrophobic nature, as shown by Tamada and Ikada.13 Therefore, the more hydrophilic and the more hydrophobic surfaces are less adhesive to biomolecules. As a strategy of surface properties modification, highly hydrophilic PET surfaces were prepared thank to helium and oxygen plasma-treatments with associated water contact angle (WCA) values around 0°.17 Immediately after the plasma-treatment, these surfaces were grafted with surfactant solutions in order to avoid any aging phenomenon. The surface properties of grafted-PET were characterized in function of various parameters of initial surfactant solution (chemical nature, composition and concentration).Influence of pluronic grafting on surface properties of the He or O2 plasma-treated PET
The chosen block copolymers, F68 and F108, have the same proportion of ethylene glycol unit (80%) but different molecular weights. The former molecular weight is around 8350 g mol−1 while the latter one is 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1. These polymers were grafted with different initial solution concentrations, [F], fixed at 0.1, 0.5, 1 and 5 g L−1. Pluronic F68 and F108 present the critical micelle concentrations (CMC) at 0.04 mM (0.334 g L−1) and 0.02 mM (0.293 g L−1) respectively and therefore; the chosen concentrations vary above and below their CMC. The effect on the surface energy of the four Pluronic concentrations was evaluated. Surface free energy (γT) and both polar (γp) and dispersive (γd) components of Pluronic F68 and F108 grafted on He and O2 plasma-treated surfaces were obtained using the Fowkes approach. Since the dispersive component can be extracted from the difference between the total and polar surface energies, only γT, γp values are given in Table 1.
600 g mol−1. These polymers were grafted with different initial solution concentrations, [F], fixed at 0.1, 0.5, 1 and 5 g L−1. Pluronic F68 and F108 present the critical micelle concentrations (CMC) at 0.04 mM (0.334 g L−1) and 0.02 mM (0.293 g L−1) respectively and therefore; the chosen concentrations vary above and below their CMC. The effect on the surface energy of the four Pluronic concentrations was evaluated. Surface free energy (γT) and both polar (γp) and dispersive (γd) components of Pluronic F68 and F108 grafted on He and O2 plasma-treated surfaces were obtained using the Fowkes approach. Since the dispersive component can be extracted from the difference between the total and polar surface energies, only γT, γp values are given in Table 1.
| Pluronic F68 | Pluronic F108 | |||||||
|---|---|---|---|---|---|---|---|---|
| a Pristine PET: γT = 43.8 ± 0.9 mJ m−2, γp = 5.4 ± 0.2 mJ m−2; He plasma-treated PET: γT = 70.9 ± 1.5 mJ m−2, γp = 49.8 ± 0.5 mJ m−2; O2 plasma-treated PET: γT = 70.9 ± 1.2 mJ m−2, γp = 50.3 ± 0.4 mJ m−2. | ||||||||
| He plasma-treated surfaces | ||||||||
| [F] | 0.012 | 0.060 | 0.120 | 0.600 | 0.007 | 0.035 | 0.070 | 0.350 |
| γT | 71.7 ± 1.1 | 70.8 ± 1.5 | 70.9 ± 0.1 | 71.6 ± 1.9 | 72.1 ± 0.2 | 72.4 ± 0.2 | 73.3 ± 0.2 | 72.8 ± 0.0 |
| γp | 37.9 ± 1.2 | 37.3 ± 1.9 | 36.9 ± 0.1 | 37.9 ± 2.3 | 39.9 ± 0.1 | 40.3 ± 0.2 | 41.0 ± 0.1 | 40.4 ± 0.2 |
| [F68] = 0.060 | [F108] = 0.035 | [C12E9] = 0.01 | [C12E9] = 0.4 | [CTAB] = 0.1 | [CTAB] = 4 | [STDC] = 0.15 | [STDC] = 7 | |
| γT | 70.8 ± 1.5 | 72.4 ± 0.2 | 73.3 ± 0.7 | 73.4 ± 0.1 | 71.0 ± 0.8 | 74.1 ± 0.1 | 72.7 ± 0.3 | 72.1 ± 0.3 |
| γp | 37.3 ± 1.9 | 40.3 ± 0.2 | 39.5 ± 0.7 | 40.6 ± 0.1 | 38.0 ± 0.7 | 40.9 ± 0.5 | 39.3 ± 0.3 | 38.2 ± 0.4 |
| γ+ | 1.8 ± 0.2 | 1.8 ± 0.4 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.3 | 1.7 ± 0.0 | 1.6 ± 0.1 | 1.4 ± 0.2 |
| γ− | 38.8 ± 3.2 | 31.7 ± 2.2 | 42.5 ± 1.6 | 43.4 ± 0.5 | 39.7 ± 2.3 | 43.6 ± 0.7 | 42.2 ± 0.9 | 41.7 ± 1.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| O2 plasma-treated surfaces | ||||||||
| [F] | 0.012 | 0.060 | 0.120 | 0.600 | 0.007 | 0.035 | 0.070 | 0.350 |
| γT | 73.3 ± 0.3 | 74.2 ± 0.1 | 70.8 ± 2.9 | 71.4 ± 2.3 | 73.2 ± 0.3 | 73.6 ± 0.1 | 72.9 ± 0.1 | 72.5 ± 0.2 |
| γp | 41.4 ± 0.1 | 39.5 ± 0.2 | 35.2 ± 3.7 | 35.9 ± 3.0 | 38.2 ± 0.4 | 39.0 ± 0.2 | 37.7 ± 0.2 | 37.6 ± 0.3 |
| [F68] = 0.060 | [F108] = 0.035 | [C12E9] = 0.01 | [C12E9] = 0.4 | [CTAB] = 0.1 | [CTAB] = 4 | [STDC] = 0.15 | [STDC] = 7 | |
| γT | 74.2 ± 0.1 | 73.6 ± 0.1 | 72.4 ± 0.1 | 70.0 ± 1.1 | 45.4 ± 2.5 | 41.2 ± 0.4 | 70.5 ± 1.7 | 72.7 ± 0.8 |
| γp | 39.5 ± 0.2 | 39.0 ± 0.2 | 39.3 ± 0.4 | 40.1 ± 2.4 | 11.8 ± 0.6 | 11.8 ± 0.5 | 39.9 ± 2.8 | 38.2 ± 1.0 |
| γ+ | 1.6 ± 0.0 | 1.7 ± 0.1 | 1.9 ± 0.0 | 1.7 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 2.4 ± 0.2 | 1.3 ± 0.2 |
| γ− | 42.6 ± 0.0 | 42.8 ± 0.7 | 40.5 ± 0.7 | 42.3 ± 3.5 | 17.7 ± 3.3 | 19.3 ± 1.3 | 39.0 ± 3.9 | 42.6 ± 2.4 |
The grafting produces a slight decrease on the surface energy values of plasma-treated surfaces, that are 75.0 mJ m−2 and 74.9 mJ m−2 for He and O2 plasma-treatments respectively. Grafting with the different concentrations of Pluronic F68 and F108 produces a similar trend in the surface energy, see Table 1. The average values of the γT for Pluronic F68 and F108 on He plasma-treated surfaces are 71.2 and 72.6 mJ m−2 respectively. On O2 plasma-treated surfaces, values are slightly higher than on He plasma-treated surfaces, 72.4 and 73.0 mJ m−2 for Pluronic F68 and F108 respectively. From the average values, it can be observed that surfaces grafted with Pluronic F108 show higher energy values (γT). The small variations observed on the surface energy values for both plasma treatments indicate no influence of the Pluronic concentration and unimer-micelle formation balance, probably explained by a surface saturation obtained at very low Pluronic concentration whatever is its molecular weight.
Influence of other surfactants grafting on surface properties of the He or O2 plasma-treated PET
Lewis acid–base interactions also play a major role on the bacterial adhesion and the formation of biofilm.18 In order to investigate such a dependence, different surfactants molecules were also grafted:C12E9 is nonionic molecule while the others are anionic (STDC) and cationic (CTAB). Two different concentrations for each surfactant were selected, above and below their CMC that means, that surfactants were on unimer and micelle form in the grafting solution.
The surface energy and their components (polar, dispersive, acid and basic) produced by three different surfactants grafted on the plasma-treated surfaces was studied (Table 1). Changes in the surfactant concentration produce small variations on the surface energy values. Moreover, all the values obtained are in the same range, around 74 mJ m−2 and 70 mJ m−2, except for O2 plasma-treated surfaces grafted with CTAB where a significant reduction is observed for the surface free energy and their polar, acid and basic components. In fact, the average energy values for He (γT = 72.5; γp = 39.3; γ− = 41.9; γ+ = 1.7 mJ m−2) and O2 (γT = 72.2; γp = 39.3; γ− = 41.8; γ+ = 1.7 mJ m−2) plasma-treated surfaces present the same trend with the exception of O2 plasma-treated surfaces grafted with CTAB (γT = 43.3; γp = 11.8; γ− = 18.5; γ+ = 0.1 mJ m−2). These results only show the influence of the surface chemistry achieved by the plasma treatment on the surfactant interaction with the surface, particularly with CTAB.
Influence of mixed surfactants grafting on surface properties of the He or O2 plasma-treated PET
The surface grafting of poly(N-isopropylacrylamide) and surfactants materials, depending on the individual proportion of each component, have been shown to form films, either homogeneous or composed of small domains of one component leading to a nano-structuration of the grafted surface.19 Such typical topography but also chemical composition may control the interactions between the substrate and the targeted biomolecule. Therefore, this procedure was also developed for the chosen components. The effect of mixed surfactant solutions on the surface energy of the plasma-treated surfaces was studied fixing the Pluronic concentration at 0.5 g L−1 ([F68] = 0.060 and [F108] = 0.035 mM) and mixing the solution at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume with the other surfactant solution. Surface free energy values and their polar, acid and basic components are shown in Table 2.
1 in volume with the other surfactant solution. Surface free energy values and their polar, acid and basic components are shown in Table 2.
| C12E9 (0.01 mM) | C12E9 (0.4 mM) | CTAB (0.1 mM) | CTAB (4 mM) | STDC (0.15 mM) | STDC (7 mM) | |
|---|---|---|---|---|---|---|
| a Pristine PET: γT = 43.8 ± 0.9 mJ m−2, γp = 5.4 ± 0.2 mJ m−2; He plasma-treated PET: γT = 70.9 ± 1.5 mJ m−2, γp = 49.8 ± 0.5 mJ m−2, γ− = 44.0 ± 0.1 mJ m−2, γ+ = 1.8 ± 0.1 mJ m−2; O2 plasma-treated PET: γT = 70.9 ± 1.2 mJ m−2, γp = 50.3 ± 0.4 mJ m−2, γ− = 43.9 ± 0.1 mJ m−2, γ+ = 1.6 ± 0.1. | ||||||
| He plasma and F68 | ||||||
| γT | 68.5 ± 0.7 | 71.8 ± 0.4 | 73.9 ± 0.2 | 73.9 ± 0.1 | 68.3 ± 0.0 | 70.0 ± 0.6 |
| γp | 33.7 ± 0.7 | 37.8 ± 0.5 | 40.6 ± 0.2 | 40.6 ± 0.2 | 33.3 ± 0.0 | 36.0 ± 0.9 |
| γ+ | 1.0 ± 0.2 | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.0 | 1.0 ± 0.1 | 1.1 ± 0.0 |
| γ− | 38.2 ± 0.4 | 41.8 ± 0.5 | 43.7 ± 0.3 | 43.9 ± 0.1 | 38.1 ± 0.5 | 40.8 ± 1.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| He plasma and F108 | ||||||
| γT | 71.7 ± 1.8 | 73.3 ± 0.2 | 69.9 ± 0.3 | 72.0 ± 0.4 | 67.4 ± 0.2 | 71.4 ± 0.5 |
| γp | 38.2 ± 2.1 | 40.5 ± 0.2 | 35.5 ± 0.8 | 38.4 ± 0.6 | 32.7 ± 0.4 | 37.4 ± 0.8 |
| γ+ | 1.8 ± 0.2 | 1.7 ± 0.0 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.1 |
| γ− | 40.0 ± 3.4 | 43.0 ± 0.4 | 37.3 ± 0.3 | 41.4 ± 1.1 | 36.8 ± 0.4 | 41.2 ± 1.5 |
| γT | 71.7 ± 1.8 | 73.3 ± 0.2 | 69.9 ± 0.3 | 72.0 ± 0.4 | 67.4 ± 0.2 | 71.4 ± 0.5 |
| γp | 38.2 ± 2.1 | 40.5 ± 0.2 | 35.5 ± 0.8 | 38.4 ± 0.6 | 32.7 ± 0.4 | 37.4 ± 0.8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| O2 plasma and F68 | ||||||
| γT | 70.8 ± 1.3 | 74.3 ± 0.1 | 44.7 ± 0.9 | 43.2 ± 2.3 | 73.7 ± 0.3 | 69.7 ± 1.7 |
| γp | 38.0 ± 0.6 | 40.5 ± 0.2 | 16.2 ± 1.6 | 10.0 ± 1.2 | 39.6 ± 0.8 | 35.4 ± 2.2 |
| γ+ | 1.4 ± 0.5 | 1.6 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 |
| γ− | 41.7 ± 3.1 | 43.7 ± 0.3 | 24.1 ± 5.6 | 18.9 ± 2.2 | 44.3 ± 1.1 | 39.4 ± 3.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| O2 plasma and F108 | ||||||
| γT | 73.2 ± 0.2 | 73.4 ± 0.1 | 51.3 ± 0.4 | 39.8 ± 1.2 | 73.6 ± 0.4 | 72.7 ± 0.1 |
| γp | 41.2 ± 0.6 | 43.7 ± 0.2 | 17.1 ± 0.6 | 13.3 ± 0.7 | 40.5 ± 0.3 | 39.4 ± 0.2 |
| γ+ | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| γ− | 43.0 ± 0.4 | 45.4 ± 0.1 | 25.5 ± 1.4 | 20.1 ± 2.9 | 43.1 ± 0.3 | 41.6 ± 0.5 |
Solutions in which the individual components present similar surface energies γT, around 72 mJ m−2, give place to similar range of values of energy. In those cases, it can be observed that the surface energy γT can achieve slightly lower values, around 68 mJ m−2, with mixed surfactants than with the solutions of the individual components. Solutions in which the individual components present different surface energies γT, around 72 and 43 mJ m−2 for Pluronic and CTAB respectively when grafted on O2 plasma-treated surfaces; it can be distinguished a predominant presence of CTAB on the surface when the grafting solution is made with Pluronic F68, since the values obtained are comparable to the values of surfaces grafted with CTAB solutions. However, when Pluronic F108 is present in the mixed solution with CTAB, it can be observed γT modulation. CTAB adduct in the Pluronic solution leads to lower surface energy (γT) values than with solutions containing only CTAB, indicating a remarkable presence of the Pluronic on the grafted surface. Such results indicate the possibility of tailoring the surface energy values of oxygen plasma-treated surfaces with values around 72 and 40 mJ m−2 by the proper concentration of the components forming the solution that means CTAB and Pluronic and explained by a strong and selective affinity between plasma-attached polar groups and surfactant. Regarding to the energy components, we remark a general reduction on the acid character for all the mixed surfactant solutions containing Pluronic F68 and, especially on the surfaces grafted with solutions containing CTAB.
Surface chemistry of the various surfactant grafted PET
The chemistry of plasma-treated surface as well as from grafted surfaces was also studied by XPS. Plasma-treated surfaces were grafted with Pluronic F68 (0.5 g L−1, 0.060 mM) and mixed solutions of Pluronic F68 and surfactants, C12E9 (0.4 mM) and CTAB (4 mM).From the survey spectra, the relative atomic concentration of the C and O elements was obtained (Table 3). PET surfaces present an O/C ratio of 0.20 that it is increased to 0.45 by both plasma treatments, indicating the incorporation of oxygen-containing groups by means of the plasma treatments. On grafted surfaces, a modification of the O/C ratio (Table 3) is observed, increasing significantly on surfaces grafted with Pluronic F68 and C12E9, meanwhile a remarkable decrease is obtained on surfaces treated with oxygen plasma, especially on surfaces grafted with solutions containing Pluronic F68 and CTAB.
| Grafted PET | O/C | C1, C–C/C–H | C2 C–O | C3 O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
C4 π → π* | C5 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| eV | % | eV | % | eV | % | eV | % | eV | % | ||
| a XPS data of pristine and He, O2 plasma-treated are given in ref. 17. | |||||||||||
| O2 plasma | |||||||||||
| PF68 | 0.42 | 285.0 | 67.9 | 286.5 | 23.2 | 289.1 | 5.0 | — | — | 287.8 | 3.9 |
| PF68/CTAB | 0.29 | 285.0 | 76.5 | 286.5 | 15.7 | 289.2 | 3.6 | — | — | 287.8 | 4.2 |
| PF68/C12E9 | 0.37 | 285.0 | 70.4 | 286.5 | 20.1 | 289.1 | 7.2 | — | — | 287.5 | 2.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| He plasma | |||||||||||
| PF68 | 0.44 | 285.0 | 51.3 | 286.5 | 28.5 | 289.1 | 11.2 | 291.1 | 2.2 | 287.8 | 6.8 |
| PF68/CTAB | 0.43 | 285.0 | 60.5 | 286.5 | 22.4 | 289.1 | 8.4 | 291.1 | 1.2 | 287.8 | 7.5 |
| PF68/C12E9 | 0.54 | 285.0 | 57.4 | 286.5 | 23.2 | 289.1 | 10.0 | 290.9 | 1.8 | 287.5 | 7.6 |
The high-resolution C1s spectrum of the pristine PET surface was decomposed into three main peaks, C1 at 285.0 eV assigned to the aliphatic carbon (C–C and C–H groups), C2 at 286.7 eV attributed to the ether carbon (C–O group) and C3 at 289.1 eV attributed to the carboxylic carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group).20 Besides, a shake-up peak is observed at 291.1 eV (peak C4), indicating the presence of aromatic rings. After helium plasma treatment, a new functional group appears around 287.8 eV (peak C5) assigned to the C
O group).20 Besides, a shake-up peak is observed at 291.1 eV (peak C4), indicating the presence of aromatic rings. After helium plasma treatment, a new functional group appears around 287.8 eV (peak C5) assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups,21–23 meanwhile no new groups were added to the surface by O2 plasma treatments. Therefore, He plasma-treatments produce a reduction of carboxylic contents (peak C3) and an increase in ester groups (peak C2). However, O2 plasma-treatment decreases the aliphatic carbon and increases both ester and carboxylic components. So, a significant difference in the carboxylic content of the PET surfaces is obtained by the different plasma-treatments. Moreover, the shake-up satellite peak did not disappear completely with the plasma treatments.
O groups,21–23 meanwhile no new groups were added to the surface by O2 plasma treatments. Therefore, He plasma-treatments produce a reduction of carboxylic contents (peak C3) and an increase in ester groups (peak C2). However, O2 plasma-treatment decreases the aliphatic carbon and increases both ester and carboxylic components. So, a significant difference in the carboxylic content of the PET surfaces is obtained by the different plasma-treatments. Moreover, the shake-up satellite peak did not disappear completely with the plasma treatments.
The different chemistry obtained on plasma-treated surfaces (drastic drop of the carboxylic content on He plasma-treated PET and a considerable increase of ether carbons on O2 plasma-treated PET), could be the reason of the different interaction of CTAB with the plasma-treated surfaces. In fact, the presence of the ether carbon could promote the interaction with CTAB.
Grafted surfaces present different trend depending on the plasma-treatment of the PET surface. For He plasma-treated surfaces, a reduction of the aliphatic carbon component (peak C1) and an increment of ether and carboxylic carbon (peaks C2 and C3 respectively) is observed. Such changes are higher on surfaces grafted with Pluronic F68 than on surfaces grafted with mixed solutions (Table 3). Regarding to the oxygen plasma-treated surfaces (Table 3), the opposite trend, an increment of the aliphatic carbon and a reduction of ether and carboxylic carbon, with surfaces grafted with the surfactant solution containing CTAB showing the higher modification. Moreover, all the grafted surfaces showed the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups (peak C5) and the shake-up peak is observed in the surfaces treated with He plasma.
O groups (peak C5) and the shake-up peak is observed in the surfaces treated with He plasma.
Surface topography of the various surfactant grafted PET
The surface topography was observed at two different scales, the first one at micrometric scale and the second one at nanometric respectively thanks to the mapping of the CA distribution of ultra-pure water picodrops and AFM images. Plasma-treated PET was showing a superhydrophilic character (WCA values around 1°). The increase in the CA values of grafted surfaces respect to the plasma-treated ones, indicates the adhesion of the surfactants on the plasma-treated surfaces. The average CA values obtained from the 64 drops deposited for the CA mapping (Fig. 1) were 8.8° ± 1.1°, 25.6° ± 2.2°, 10.3° ± 3.3° and 7.6° ± 2.5° for Pluronic F68, Pluronic F68/STDC, Pluronic F68/C12E9 and Pluronic F68/CTAB grafted on He plasma-treated surfaces respectively. On O2 plasma-treated surfaces, the average CA values obtained were 8.9° ± 1.6°, 26.5° ± 4.9°, 32.8° ± 2.0° and 67.6° ± 6.9° for Pluronic F68, Pluronic F68/STDC, Pluronic F68/C12E9 and Pluronic F68/CTAB respectively. On example of the CA map is given in Fig. 1, where scale from 0° to 40° has been used for He plasma-treated surfaces and from 0° to 80° for O2 plasma-treated surfaces. CA maps indicate a quite high homogeneity of the grafting on the surface and consequently, a good adherence of the surfactants on the surface.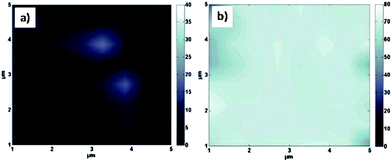 | ||
| Fig. 1 Examples of contact angle mapping for (a) He plasma-treated surfaces grafted with Pluronic F68/CTAB and (b) O2 plasma-treated surfaces grafted with Pluronic F68/CTAB. | ||
The surface topography of grafted substrates at nanoscale was studied by means of AFM (Fig. 2), showing a decrease of the average surface roughness (Ra) with grafting processes. In fact, the roughness value decreases from 1.6 and 3.6 nm for He and O2 plasma-treated PET respectively to values ranging between 0.7–0.89 nm. From the height AFM images (Fig. 2), it can be observed that after the grafting of any surfactant, features like nanodots are formed leading to a rather smooth nanostructured surface.
 | ||
| Fig. 2 Height AFM images (2 × 2 μm) and roughness of surfaces grafted with (a) Pluronic F68, (b) Pluronic F68/CTAB (0.1 mM), Pluronic F68/CTAB (4 mM). | ||
Anti-fouling properties of the various surfactant grafted PET
The grafting stability in an aqueous media, shown in ref. 17 allows the use of grafted surfaces for biological applications. Therefore, the anti-fouling character of surfaces grafted with Pluronic (F68 and F108) and mixed solutions of Pluronic with surfactants was studied by confocal microscopy and illustrated for protein adhesion (Fig. 3 and 4). | ||
| Fig. 3 158 × 158 μm2 confocal microscope image of a surface where the upper part of the sample is the pristine PET and the lower part has been treated with O2 plasma and grafted with Pluronic F108. | ||
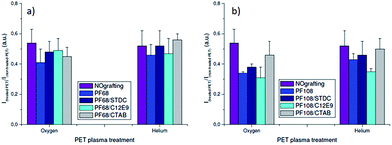 | ||
| Fig. 4 Intensity ratio (Igrafted/Iblank) for surfaces grafted with (a) Pluronic F68 and (b) Pluronic F108. | ||
The intensity from confocal images showed higher values on non-treated surfaces, indicating a higher adhesion of proteins on pristine PET. The intensity ratios obtained a reduction of the signal intensity from plasma-treated surfaces and surfaces grafted with surfactants.
In Fig. 3, it can be observed the higher intensity signal on pristine surface (upper part of the image) and the reduction of the signal on the O2 plasma and grafted with Pluronic F108 surface (low part of the image). Indeed, intensity ratios were 0.54 and 0.52 for O2 and He plasma treatments respectively, indicating a reduction of almost a 50% in the bioadhesive character with both plasma treatments.
Such antifouling character was increased by grafting of surfactants on the plasma treated PET as can be seen in Fig. 4. Particularly, the intensity ratios obtained from O2 plasma-treated surfaces grafted with solutions containing are lower than 0.4, which means more than a 60% reduction of their bioadhesive character. As reference for these results, plasma-treated PET surfaces were covered with PEG (used without dilution) during 3 min, and then were extensively rinsed with distilled water to removed not well attached molecules. These samples were incubated with proteins following the same protocol used with surfaces grafted with Pluronic solutions. The intensity ratios obtained for O2 and He plasma-treated surfaces grafted with PEG were around 0.29 ± 0.02 and 0.28 ± 0.05 respectively, that means a reduction of the bioadhesive character of the treated surfaces higher than 70%. Which indicates a good antifouling character of the surfaces treated with Pluronic, particularly Pluronic F108 on oxygen treated surfaces, since the intensity ratios are slightly higher than the obtained with the PEG, a polymer broadly used by its anti-fouling character. However, the adhesion decrease is less pronounced with He plasma-treated and F68-grafted surfaces. This cannot be explained by different chemical composition or roughness of the surface since most of the tested surfaces are closed together. It may due to the possible molecular weight and surface charge effects. Works are still under progress.
The effect on protein adhesion of the aging of plasma-treated surfaces was checked. O2 and He plasma-treated samples, as well as plasma-treated surfaces grafted with Pluronic F108 and STDC mixed solutions were kept in air and 20 °C for 28 days. After this period of time, the protein bioadhesion steps were carried out. Intensity ratios around 0.7 were obtained from the confocal images showing an increment of adhesion of protein on aged surfaces compared with surfaces incubated the same day that the surface treatment was completed (plasma and grafting steps). The surface chemistry is shown to be altered in this period of time with a water contact angle increase of around 10–15°. This surface ageing could explain the decrease of non-fouling properties. Nevertheless, intensity ratios lower than non treated surfaces were still obtained showing a reduction of 30% of the bioadhesive character.
Conclusions
In this study, we have explored the enhancement of the antifouling properties of grafted plasma-treated PET surfaces. For that, two different gases, helium and oxygen, were used to obtain superhydrophilic surfaces with different surface chemistries. Surfaces were then grafted with solutions containing surfactants. A good adherence and high homogeneity of the surfactants on the surface was observed from the CA distribution maps.The chemical composition and the surface energy of grafted surfaces were analyzed. XPS results showed two differentiated trends on the chemistry of the grafting, dependent on the plasma treatment followed. For He plasma-treated surfaces, a reduction of the aliphatic carbon component and an increment of ether and carboxylic carbon is observed. The opposite trend is observed with O2 plasma-treated surfaces. Besides, surface energy data showed similar energy values on the plasma-treated PET grafted with the different surfactant solutions, except for oxygen plasma-treated surfaces grafted with solutions containing CTAB. In fact, oxygen plasma-treated surfaces grafted with solutions containing Pluronic F108 and CTAB showed surface energy values ranging from 72 to 40 mJ m−2, indicating the possibility of tailoring the surface energy by modifying the concentration of Pluronic and CTAB in the grafting solution. These modified surfaces were also dipped in different water solutions containing either P. Aeruginosa or L. monocytogenes bacterias in order to evaluate their bacterial–adhesion properties. It appears that the anti-fouling character in function of the bacteria is less pronounced than for proteins but is only dependent on the Lewis base character of the modified surfaces; the stronger is, the greater the nonadherence is. This study is still under progress.
The grafting stability in water allowed carrying out the protein immobilization.17 Confocal images showed a higher adhesion of proteins on non treated surfaces. A reduction of the 50% on the bioadhesion was observed on plasma-treated surfaces grafted with solutions containing Pluronic F68, meanwhile a reduction around the 60% was achieved when the solution contained Pluronic F108, indicating a better antifouling character when surfaces are grafted with polymers of longer chains. A reduction on the antifouling properties achieved with these techniques was observed due to the aging of plasma-treated surfaces. Nevertheless, despite the aging a reduction of 30% of the bioadhesive character was observed.
Acknowledgements
This work was supported by ANR French agency in the frame of SANBACT project.References
- S. Burkert, M. Kuntzsch, C. Bellmann, P. Uhlmann and M. Stamm, Appl. Surf. Sci., 2009, 255(12), 6256–6261 CrossRef CAS PubMed.
- S. Han, Y. Lee, H. Kim, G. H. Kim, J. Lee, J. H. Yoon and G. Kim, Surf. Coat. Technol., 1997, 93(2–3), 261–264 CrossRef CAS.
- C. M. Chan, T. M. Ko and H. Hiraoka, Surf. Sci. Rep., 1996, 24(1–2), 1–54 CrossRef CAS.
- J. Friedrich, L. Wigant, W. Unger, A. Lippitz and H. Wittrich, Surf. Coat. Technol., 1998, 98(1–3), 879–885 CrossRef CAS.
- M. Collaud-Coen, R. Lehmann, P. Groening and L. Schlapbach, Appl. Surf. Sci., 2003, 207(1–4), 276–286 CrossRef CAS.
- G. Poletti, F. Orsini, A. Raffaele-Addamo, C. Riccardi and E. Selli, Appl. Surf. Sci., 2003, 219(3–4), 311–316 CrossRef CAS.
- M. M. Hossain, D. Hegemann, A. S. Herrmann and P. Chabrecek, J. Appl. Polym. Sci., 2006, 102(2), 1452–1458 CrossRef CAS.
- N. De Geyter, R. Morent, C. Leys, L. Gengembre and E. Payen, Surf. Coat. Technol., 2007, 201(16–17), 7066–7075 CrossRef CAS PubMed.
- S. M. Pelagade, N. L. Singh, A. Qureshi, R. S. Rane, S. Mukherjee, U. P. Deshpande, V. Ganesan and T. Shripathi, Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2012, 289, 34–38 CrossRef CAS PubMed.
- N. Krstulović, I. Labazan, S. Milošević, U. Cvelbar, A. Vesel and M. Mozetič, J. Phys. D: Appl. Phys., 2006, 39, 3799–3804 CrossRef.
- M. Gheorghiu, F. Arefi, J. Amouroux, G. Placinta, G. Popa and M. Tatoulian, Plasma Sources Sci. Technol., 1997, 6(1), 8–19 CrossRef CAS.
- D. Papakonstantinou, E. Amanatides, D. Mataras, V. Ioannidis and P. Nikolopoulos, Plasma Processes Polym., 2007, 4(S1), S1057–S1062 CrossRef.
- Y. Tamada and Y. Ikada, Polymer, 1993, 34, 2208–2212 CrossRef CAS.
- Plasma Chemistry, ed. A. Friedman, Cambrodge University Press, 2008 Search PubMed.
- M. R. Nejadnik, H. C. van der Mei, W. Norde and H. J. Busscher, Biomaterials, 2008, 29, 4117–4121 CrossRef CAS PubMed.
- Q. N. Fu, K. X. Zhi, J. H. Xiao, Y. Peng and W. Jian, Langmuir, 2003, 19, 9889–9895 CrossRef.
- M. J. Perez-Roldan, D. Debarnot and F. Poncin-Epaillard, RSC Adv., 2014, 4(59), 31409–31415 RSC.
- R. Bos, H. C. van der Mei and H. J. Busscher, FEMS Microbiol. Rev., 1999, 23, 179–230 CAS.
- T. Vrlinic, D. Debarnot, M. Mozetic, A. Vesel, J. Kovac, A. Coudreuse, G. Legeay and F. Poncin-Epaillard, J. Colloid Interface Sci., 2011, 362, 300–310 CrossRef CAS PubMed.
- H. Hantsche, High resolution XPS of organic polymers, the scienta ESCA300 database. ed. G. Beamson and D. Briggs, Wiley, Chichester 1992, p. 295 Search PubMed.
- J. R. Friedrich, I. Loeschcke, H. Frommelt, H. D. Reiner, H. Zimmermann and P. Lutgen, Polym. Degrad. Stab., 1991, 31(1), 97–114 CrossRef CAS.
- Y. Ren, C. Wang and Y. Qiu, Surf. Coat. Technol., 2008, 202(12), 2670–2676 CrossRef CAS PubMed.
- P. A. Charpentier, A. Maguire and W. K. Wan, Appl. Surf. Sci., 2006, 252(18), 6360–6367 CrossRef CAS PubMed.
Footnote |
| † Present address: CIC NanoGUNE Consolider, Tolosa Hiribidea 76, 20018 Donostia-San Sebastian, Spain. |
| This journal is © The Royal Society of Chemistry 2014 |




