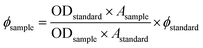A quinazoline derivative as quick-response red-shifted reporter for nanomolar Al3+ and applicable to living cell staining†
Manjira Mukherjeea,
Buddhadeb Sena,
Siddhartha Pala,
Samya Banerjeeb,
Somenath Lohara and
Pabitra Chattopadhyay*a
aDepartment of Chemistry, Burdwan University, Golapbag, Burdwan-713104, West Bengal, India. E-mail: pabitracc@yahoo.com
bDepartment of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore, 560012, India
First published on 6th November 2014
Abstract
A newly synthesized and structurally characterized quinazoline derivative (L) has been shown to act as a quick-response chemosensor for Al3+ with a high selectivity over other metal ions in water–DMSO. In the presence of Al3+, L shows a red-shifted ratiometric enhancement in fluorescence as a result of internal charge transfer and chelation-enhanced fluorescence through the inhibition of a photo-induced electron transfer mechanism. This probe detects Al3+ at concentrations as low as 1.48 nM in 100 mM HEPES buffer (DMSO–water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) at biological pH with a very short response time (15–20 s). L was applied to biological imaging to validate its utility as a fluorescent probe for monitoring Al3+ ions in living cells, illustrating its value in practical environmental and biological systems.
9 v/v) at biological pH with a very short response time (15–20 s). L was applied to biological imaging to validate its utility as a fluorescent probe for monitoring Al3+ ions in living cells, illustrating its value in practical environmental and biological systems.
Introduction
The development of novel quick-response chemosensors for trace amounts of biologically active metal ions is currently a very active field of research as a result of their potential applications in the life sciences, medicine, chemistry and biotechnology.1 Aluminium is the most abundant trace element in the Earth's crust (8.3% by weight) and is used extensively,2 resulting in an increase in the concentration of Al3+ in food. Aluminium compounds are also frequently used in pharmaceutical drugs in human and veterinary medicine and in cosmetics, including antiperspirants.3 Aluminium salts are, however, neurotoxic and are suspected to induce Parkinson's disease and Alzheimer's disease. They may even induce genotoxic effects and inhibit the repair of radiation-induced lesions in human peripheral blood lymphocytes.4 After absorption, aluminium is generally distributed and accumulated to all body tissues in humans and animals and may give rise to colic, rickets, gastrointestinal problems, interference with the metabolism of calcium, extreme nervousness, anaemia, headaches, decreased liver and kidney function, memory loss, speech problems, softening of the bones, aching muscles and even lung cancer, as it is reported to obstruct the respiratory chain by interfering with the functioning of iron–sulfur proteins.5 The World Health Organization advises an average daily human intake of Al3+ of around 3–10 mg kg−1. The tolerable weekly dietary intake is 7 mg kg−1 body weight.6As a result of these negative effects on health, the detection of aluminium ions is very important. A lack of spectroscopic characteristics and the poor coordination ability of Al3+ ions7 have stimulated efforts to develop an Al3+ ion-selective fluorescence probe. Fluorescence techniques offer significant advantages over other methods of detection.8–10 Most of the reported Al3+ sensors based on single-point chelation-enhanced fluorescence (CHEF)/photo-induced electron transfer11 suffer from interference by Fe3+ and Cu2+ ions, require tedious synthetic methods and are poorly soluble in water.12 Ratiometric CHEF-based Al3+-selective chemosensors have rarely been reported,13 although there are many experimental advantages of ratiometric fluorescence signalling that can provide built-in corrections for environmental effects and stability under illumination.14
We have developed an efficient ratiometric chemosensor using 6-[2-(2-nitro-phenyl)-vinyl]-5,6-dihydro-benzo[4,5]imidazo[1,2-c]quinazoline (L) for the selective sensing of Al3+ ions in DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) based on a CHEF mechanism. This simple and easy to synthesize chemosensor has a high emission yield, excellent photo-stability and significant fluorescent behaviour in the visible region. The sensor is not affected by the presence of an excess of other competitive metal ions, including all the alkali and alkaline earth metal ions, as a result of the selective formation of L′–Al species.
9 v/v) based on a CHEF mechanism. This simple and easy to synthesize chemosensor has a high emission yield, excellent photo-stability and significant fluorescent behaviour in the visible region. The sensor is not affected by the presence of an excess of other competitive metal ions, including all the alkali and alkaline earth metal ions, as a result of the selective formation of L′–Al species.
Experimental
Materials and physical measurements
The analytical-reagent grade solvents and other reagent-grade chemicals used in this study were purchased from commercial sources and were used as received. Elemental analyses (C, H and N) were carried out on a PerkinElmer 2400 CHN elemental analyzer. UV-1800 and Prestige-21 spectrophotometers (Shimadzu, Japan) were used for recording the electronic and IR spectra, respectively. 1H-NMR and 13C-NMR spectra were obtained using a JEOL 400 spectrometer with DMSO-d6. A Qtof Micro YA263 mass spectrometer was used to record the electrospray ionization (ESI) mass spectra. A Systronics Model 335 digital pH meter was used to measure the pH of the solution and the pH was adjusted using either a 50 mM HCl or NaOH solution. Steady-state fluorescence emission and excitation spectra were measured using a Hitachi-4500 spectrofluorimeter. Time-resolved fluorescence lifetime measurements were obtained with a TCSPC instrument (PTI, USA) using a sub-nanosecond pulsed LED source (370 nm with a pulse width of 600 ps full width at half maximum) (PicoQuant, Germany) operating at a high repetition rate of 10 MHz and driven by a PDL 800-B driver (PicoQuant). The LED profile was measured at an excitation wavelength of 370 nm with a band pass of 3 nm using Ludox as the scatterer. The collected emission from the sample was at a right angle to the direction of the excitation beam, keeping a magic angle polarization of 54.71 and a resolution of 146 ps per channel. Felix 32 data analysis software, in which reduced w2 and weighted residuals serve as parameters for goodness-of-fit, was used to fit the data to multi-exponential functions after deconvolution of the instrument response function by an iterative reconvolution technique.The luminescence properties of L were examined in water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The pH was studied in 100 mM HEPES buffer solution by adjusting the pH with HCl or NaOH. The in vivo study was carried out at a biological pH of about 7.4 in 100 mM HEPES buffer solution. The stock solutions (about 10−2 M) for the study of the selectivity of L towards different metal ions were prepared using nitrate salts (Na+, K+, Cu2+, Cr3+, Pb2+, Cd2+ and Ag+), acetate salts (Mn2+ and Zn2+), chloride salts (Co2+, Ni2+, Ca2+, Hg2+, Mg2+, Fe3+) and Fe2+ sulphate in water–DMSO (9
1 v/v). The pH was studied in 100 mM HEPES buffer solution by adjusting the pH with HCl or NaOH. The in vivo study was carried out at a biological pH of about 7.4 in 100 mM HEPES buffer solution. The stock solutions (about 10−2 M) for the study of the selectivity of L towards different metal ions were prepared using nitrate salts (Na+, K+, Cu2+, Cr3+, Pb2+, Cd2+ and Ag+), acetate salts (Mn2+ and Zn2+), chloride salts (Co2+, Ni2+, Ca2+, Hg2+, Mg2+, Fe3+) and Fe2+ sulphate in water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). In the study of selectivity, the amount of metal ions was 100× that of the probe used. Fluorescence titration with aluminium nitrate was performed in water–DMSO (9
1 v/v). In the study of selectivity, the amount of metal ions was 100× that of the probe used. Fluorescence titration with aluminium nitrate was performed in water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) by varying the metal concentration from 0 to 100 μM; the concentration of L was 10 μM.
1 v/v) by varying the metal concentration from 0 to 100 μM; the concentration of L was 10 μM.
Preparation of 6-[2-(2-nitro-phenyl)-vinyl]-5,6-dihydro-benzo[4,5]imidazo[1,2-c]quinazoline (L)
2-Nitro-cinnamaldehyde (1.77 g, 10.0 mmol) in 25 mL of ethanol was added dropwise to an ethanolic solution of 2-(2-aminophenyl)benzimidazole (2.09 g, 10.0 mmol) (25 mL) at room temperature under a nitrogen atmosphere. The resulting mixture was refluxed for 6.0 h. The slightly yellow precipitate of compound (L) was collected by filtration after reducing the solvent via slow evaporation. Single crystals of the compound were obtained from the ethanolic solution.C22H16N4O2. Found: C, 71.94; H, 4.29; N, 15.76. Calculated: C, 71.73; H, 4.38; N, 15.21. ESI-MS [M + H]+: m/z 369.1332 (100%) (calculated m/z 369.1273). IR (KBr, cm−1): νNH 3078.39, νCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N 1631.78. 1H-NMR (400 MHz DMSO-d6): δ 7.88–7.86 (m, 2H), 7.63–7.59 (m, 3H), 7.52 (t, 1H, J = 7.64), 7.46–7.42 (m, 2H), 7.23 (t, 1H, J = 6.88), 7.18–7.09 (m, 3H), 6.88 (d, 1H, J = 8.4), 6.79 (t, 1H, J = 7.64), 6.70 (dd, 1H, J = 7.62), 6.51–6.40 (q, 1H, J1 = 15.28, J2 = 7.6). 13C-NMR (400 MHz, DMSO-d6): δ 149.09, 145.36, 145.27, 136.75, 134.62, 133.43, 130.83, 130.77, 130.48, 130.22, 129.79, 129.28, 127.21, 126.84, 126.51, 125.44, 119.98, 116.99, 115.33, 113.66, 105.91, 67.91. Yield, 90%.
N 1631.78. 1H-NMR (400 MHz DMSO-d6): δ 7.88–7.86 (m, 2H), 7.63–7.59 (m, 3H), 7.52 (t, 1H, J = 7.64), 7.46–7.42 (m, 2H), 7.23 (t, 1H, J = 6.88), 7.18–7.09 (m, 3H), 6.88 (d, 1H, J = 8.4), 6.79 (t, 1H, J = 7.64), 6.70 (dd, 1H, J = 7.62), 6.51–6.40 (q, 1H, J1 = 15.28, J2 = 7.6). 13C-NMR (400 MHz, DMSO-d6): δ 149.09, 145.36, 145.27, 136.75, 134.62, 133.43, 130.83, 130.77, 130.48, 130.22, 129.79, 129.28, 127.21, 126.84, 126.51, 125.44, 119.98, 116.99, 115.33, 113.66, 105.91, 67.91. Yield, 90%.
Preparation of the aluminium(III) complex (L′–Al species)
Solid aluminium(III) nitrate nonahydrate (375 mg, 1.0 mmol) was added to a methanolic solution of L (368.0 mg, 1.0 mmol) and the reaction mixture was stirred at ambient temperature for 6.0 h. The resulting solution was then evaporated slowly at room temperature. After a few days, a deep yellow complex was obtained by washing thoroughly with cold methanol and water and then drying in vacuo.C22H18AlN7O12. Found: C, 44.21; H, 3.01; N, 16.96. Calculated: C, 44.08; H, 3.03; N, 16.36. IR (cm−1): νNO3 1341. ESI-MS in methanol [M + Na]+: m/z 622.1861 (observed with 8% abundance) (calculated m/z 622.0727), where M = [Al(L′)(ONO2)3(H2O)]. 1H-NMR (δ, ppm in DMSO-d6): 8.07 (s, 1H), 7.92–7.73 (m, 3H), 7.65–7.62 (m,1H), 7.60 (d, 1H, J = 7.64), 7.51–7.37 (m, 5H), 7.23 (d, 1H, J = 15.28), 7.00 (d, 1H, J = 7.6), 6.84 (d, 1H, J = 7.6), 6.78 (t, 1H, J = 7.64), 6.51–6.42 (q, 1H, J1 = 15.28, J2 = 7.6). 13C-NMR (400 MHz DMSO-d6): δ 152.08, 149.07, 147.31, 144.80, 143.79, 134.45, 133.60, 132.75, 132.11, 130.87, 130.38, 129.50, 127.49, 125.77, 125.34, 123.38, 123.23, 119.62, 119.40, 116.18, 112.75, 111.60. Yield, 75%.
X-ray data collection and structural determination
Single crystals were obtained from the solution of L in methanol after slow evaporation. X-ray data were collected on a Bruker Apex-II CCD diffractometer using MoKα (λ = 0.71069) radiation. The data were corrected for Lorentz and polarization effects and empirical absorption corrections were applied using the SADABS program (Bruker). A total of 8503 reflections were measured, of which 3602 were independent and 1282 were observed [I > 2σ(I)]. The structure was solved by direct methods using SIR-92 (ref. 15) and refined by full-matrix least-squares refinement methods based on F2 using SHELX-97.16 All non-hydrogen atoms were refined anisotropically. All calculations were performed using the Wingx package.17 Table 1 lists the refined crystallographic data.| Empirical formula | C22H16N4O2 |
| Formula weight | 368.39 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 10.390(5) |
| b (Å) | 16.287(5) |
| c (Å) | 11.124(5) |
| α = β | 90° |
| γ | 105.725(5) |
| Volume (Å3) | 1812.0(13) |
| Z | 4 |
| ρcalc (g cm−3); μ (mm−1) | 1.350; 0.090 |
| F(000) | 768.0 |
| θ range (deg) | 2.380 to 19.979° |
| Reflections collected | 1681 |
| Reflections independent | 1220 |
| Final R indices [I > 2σ(I)] | R = 0.10160, wR2 = 0.3523 |
Preparation of cell and in vitro cellular imaging with L
HeLa and MCF-7 cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 μg mL−1 penicillin, 100 μg mL−1 streptomycin and 2 mM Glutamax at 37 °C in a humidified incubator under 5% CO2. The adherent cultures were grown as a monolayer and passed once every 4–5 days by trypsinizing with 0.25% trypsin–EDTA. MCF-7 and HeLa cells (4 × 104 cells per mm2), plated on cover slips, were incubated with L (10, 5 and 2 μM, 1% DMSO) for 30 min. After washing with 50 mM phosphate buffer (pH 7.4) containing 150 mM NaCl (PBS), the required volumes of aluminium nitrate stock solution in DMSO were added such that the final concentration of aluminium nitrate was adjusted to 2.0, 5.0 and 10.0 μM (1% DMSO) and incubated for 30 min. The cells were fixed with 4% paraformaldehyde for 10 min at room temperature. After washing with PBS, the cells were mounted in 90% glycerol solution containing Mowiol, an anti-fade reagent, and sealed. Images were obtained using an Apotome fluorescence microscope (Carl Zeiss, Germany) with an oil immersion lens at 63× magnification. The images were analysed using AxioVision Rel 4.8.2 (Carl Zeiss) software.18Cell cytotoxicity assay
The photo-cytotoxicity of the ligand was studied in MCF-7 cells using the MTT assay. This assay is based on the ability of the mitochondrial dehydrogenases of viable cells to cleave the tetrazolium rings of MTT to form dark purple membrane-impermeable crystals of formazan that can be measured at 540 nm after solubilization in DMSO.19 Approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were plated in 96-well culture plates in DMEM containing 10% FBS (10% DMEM). After incubation overnight at 37 °C in a CO2 incubator, the solutions containing various concentrations of L in 1% DMSO were added to the cells and incubation was continued for 8 h in the dark. After replacing the medium with fresh DMEM–FBS, incubation was continued for a further 16 h in the dark. Finally, 25 μL of 4 mg mL−1 MTT solution in PBS were added to each well and incubated for an additional 3 h. After discarding the culture medium, 200 μL of DMSO were added to dissolve the formazan crystals and the absorbance was measured at 540 nm using a Biorad ELISA plate reader. The cytotoxic effect of L was measured from the absorbance ratio of the treated cells and the control cells. Non-linear regression analysis using Graph Pad Prism software was used to determine the IC50 values.
000 cells were plated in 96-well culture plates in DMEM containing 10% FBS (10% DMEM). After incubation overnight at 37 °C in a CO2 incubator, the solutions containing various concentrations of L in 1% DMSO were added to the cells and incubation was continued for 8 h in the dark. After replacing the medium with fresh DMEM–FBS, incubation was continued for a further 16 h in the dark. Finally, 25 μL of 4 mg mL−1 MTT solution in PBS were added to each well and incubated for an additional 3 h. After discarding the culture medium, 200 μL of DMSO were added to dissolve the formazan crystals and the absorbance was measured at 540 nm using a Biorad ELISA plate reader. The cytotoxic effect of L was measured from the absorbance ratio of the treated cells and the control cells. Non-linear regression analysis using Graph Pad Prism software was used to determine the IC50 values.
Results and discussion
Synthesis and characterization
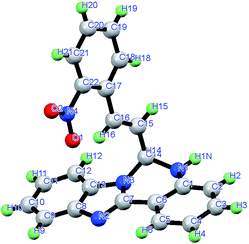
The organic moiety (L) was prepared by condensing an ethanolic solution of 2-(2-aminophenyl)benzimidazole with 2-nitro cinnamaldehyde in an equimolar ratio (Scheme 1). The physico-chemical and spectroscopic analyses and detailed structural analysis by single-crystal X-ray crystallography support the formulation of L shown in Scheme 1. L is soluble in common polar organic solvents and sparingly soluble in water. The peaks obtained in the 1H-NMR spectrum and the 13C-spectrum of L were assigned and these agree with the structural formula of L in the solution state (Fig. S1 and S2†). The ESI mass spectrum of the compound in methanol shows a peak at m/z 369.1332 with 100% abundance assignable to [M + H]+ (calculated value m/z 369.1273), where M = molecular weight of L (Fig. S3†). The IR spectrum of L shows the characteristic stretching of νN–H and νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds (Fig. S4†). Fig. 1 is an ORTEP view of probe L with the atom numbering scheme. The crystallographic data and bond parameters (selected bond distances and angles) are listed in Tables 1 and 2, respectively. The bond lengths reported in Table 2 indicate that the C14–N3 bond distance (1.444 Å) is shorter than that of C1–N1 (1.448 Å), but both values are shorter than the C14–N1 (1.458 Å) bond.
N bonds (Fig. S4†). Fig. 1 is an ORTEP view of probe L with the atom numbering scheme. The crystallographic data and bond parameters (selected bond distances and angles) are listed in Tables 1 and 2, respectively. The bond lengths reported in Table 2 indicate that the C14–N3 bond distance (1.444 Å) is shorter than that of C1–N1 (1.448 Å), but both values are shorter than the C14–N1 (1.458 Å) bond.
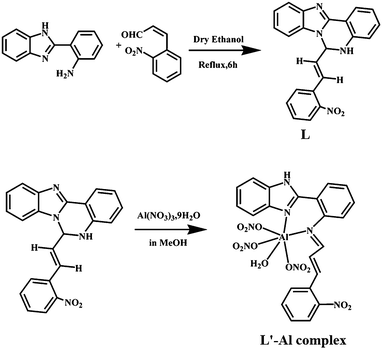 | ||
| Scheme 1 Schematic representation of the synthesis of the probe L and the corresponding Al(III) complex. | ||
| Bond length (Å) | |
| N1–C1 | 1.448(11) |
| N1–C14 | 1.458(10) |
| N2–C7 | 1.262(10) |
| N2–C8 | 1.479(12) |
| N3–C7 | 1.301(10) |
| N3–C13 | 1.484(10) |
| N3–C14 | 1.444(10) |
| C15–C16 | 1.245(9) |
| Bond angles (°) | |
| C1 N1 C14 | 121.1(7) |
| C7 N2 C8 | 100.5(8) |
| C7 N3 C14 | 128.8(8) |
| C7 N3 C13 | 103.3(8) |
| C14 N3 C13 | 125.7(8) |
| C2 C1 N1 | 123.2(9) |
To establish the formation of the L′–Al species, the solid state complex was isolated from the reaction of aluminium(III) nitrate and L in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in methanol medium with stirring. The complex is soluble in methanol, DMSO and acetonitrile. The peaks obtained in the 1H-NMR spectrum were assigned and agree with the structural formula of the complex with L′–Al as [Al(L′)(NO3)3(H2O)] (Fig. S5 and S6†). The IR spectrum of the L′–Al species shows the characteristic stretching frequency of the NO3 group (Fig. S7†). The ESI mass spectrum of the complex in methanol shows a peak at m/z 622.1861 with 8% abundance, assignable to [M + Na]+ (calculated value m/z 622.0727), where M = [Al(L′)(NO3)3(H2O)] (Fig. S8†). During the reaction with Al3+ ions, a [1,5] sigmatropic-type shift10d of L occurred prior to metal coordination (Scheme 2), giving in situ L′ which behaved as a bidentate neutral ligand to form [Al(L′)(NO3)3(H2O)].
1 molar ratio in methanol medium with stirring. The complex is soluble in methanol, DMSO and acetonitrile. The peaks obtained in the 1H-NMR spectrum were assigned and agree with the structural formula of the complex with L′–Al as [Al(L′)(NO3)3(H2O)] (Fig. S5 and S6†). The IR spectrum of the L′–Al species shows the characteristic stretching frequency of the NO3 group (Fig. S7†). The ESI mass spectrum of the complex in methanol shows a peak at m/z 622.1861 with 8% abundance, assignable to [M + Na]+ (calculated value m/z 622.0727), where M = [Al(L′)(NO3)3(H2O)] (Fig. S8†). During the reaction with Al3+ ions, a [1,5] sigmatropic-type shift10d of L occurred prior to metal coordination (Scheme 2), giving in situ L′ which behaved as a bidentate neutral ligand to form [Al(L′)(NO3)3(H2O)].
The formation of the L′–Al species through a [1,5] sigmatropic-type shift is also clearly supported by the 13C-NMR spectra of L and the L′–Al species. Here a peak at δ 67.91 (carbon atom marked as ‘v’ in Fig. S2†), attributable to the sp3 carbon atom in L, is shifted downfield at δ = 152.08 in the spectrum of the L′–Al species (Fig. S6†) as a result of the change of the sp3 carbon to an sp2 carbon by the formation of an imine-type carbon (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). All the corresponding peaks of the carbon atoms were present with the usual changes, which confirms the presence of L′ in the L′–Al species.
N). All the corresponding peaks of the carbon atoms were present with the usual changes, which confirms the presence of L′ in the L′–Al species.
Spectral characteristics
Absorption study
The UV-visible spectrum of L showed characteristic absorption bands at about 289 nm (ε = 1.744 × 104), 300 nm (ε = 1.64 × 104) and 345 nm (ε = 1.432 × 104) attributable to intramolecular π–π* and n–π* transitions. In the UV-visible titration, the addition of the solution of Al3+ ions to the colourless solution of L in DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) in HEPES buffer (0.1 M, pH 7.4) at 27 °C, a new peak appeared at 394 nm as a result of the formation of the blue colour of the resulting solution. The peak in the UV region at 345 nm in the absorption spectrum of the probe gradually decreased with the addition of Al3+ ions. A new peak was generated at around 396 nm with a 49 nm red shift through an isosbestic point at 377 nm (Fig. 2) as a result of the formation of the coloured Al(III) complex of the probe in the solution state.
9 v/v) in HEPES buffer (0.1 M, pH 7.4) at 27 °C, a new peak appeared at 394 nm as a result of the formation of the blue colour of the resulting solution. The peak in the UV region at 345 nm in the absorption spectrum of the probe gradually decreased with the addition of Al3+ ions. A new peak was generated at around 396 nm with a 49 nm red shift through an isosbestic point at 377 nm (Fig. 2) as a result of the formation of the coloured Al(III) complex of the probe in the solution state.
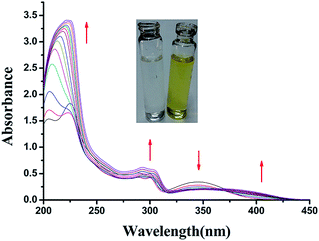 | ||
Fig. 2 UV-visible titration spectra of L with Al(III) ions (0, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15 μM, respectively) in 100 mM HEPES buffer (DMSO–water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) at 27 °C. 9 v/v) at 27 °C. | ||
Emission study
The fluorescence spectra of L displayed a very weak emission at 430 nm (λex = 380 nm) (Fig. S9†). The gradual addition of Al3+ ions (0–15 μM) to L (10 μM) causes a gradual decrease in the fluorescence intensity at 430 nm with a concomitant increase in a new band at about 476 nm through an iso-emissive point at 436 nm (Fig. 3). The weak emission of free L at 430 nm in DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) is attributed to the internal charge transfer (ICT) process. This was established by an experiment in which the bathochromic shift of the emission of L with increasing solvent polarity was recorded (Fig. S10†). L undergoes a solvent-assisted 1,5-σ tropic shift, leading to a more conjugated benzimidazole derivative with a more chelating environment at the ICT acceptor site (Scheme 1).10d With the gradual addition of Al3+ ions to L in DMSO–water (1
9 v/v) is attributed to the internal charge transfer (ICT) process. This was established by an experiment in which the bathochromic shift of the emission of L with increasing solvent polarity was recorded (Fig. S10†). L undergoes a solvent-assisted 1,5-σ tropic shift, leading to a more conjugated benzimidazole derivative with a more chelating environment at the ICT acceptor site (Scheme 1).10d With the gradual addition of Al3+ ions to L in DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v), a red shift of the emission from 430 to 476 nm occurred as a result of the binding of Al3+ at the ICT acceptor site (the imine nitrogen end) of L.20 The coordination of Al3+ ions ended the molecular flexibility and the vibration out of the plane of the fluorophore, which gives rise to the enhancement in emission at 476 nm through the CHEF process (Scheme 2).
9 v/v), a red shift of the emission from 430 to 476 nm occurred as a result of the binding of Al3+ at the ICT acceptor site (the imine nitrogen end) of L.20 The coordination of Al3+ ions ended the molecular flexibility and the vibration out of the plane of the fluorophore, which gives rise to the enhancement in emission at 476 nm through the CHEF process (Scheme 2).
 | ||
Fig. 3 Fluorescence titration of L with incremental addition of Al(III) ions (0, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15 μM, respectively) in 100 mM HEPES buffer (DMSO–water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) at 27 °C. 9 v/v) at 27 °C. | ||
The ratiometric enhancement at 476 nm (about 11.8 times) is in agreement with a 10× increase in the quantum yield. L has a quantum yield of Φ = 0.069, but the emission intensity gradually increases with the increase in added Al3+ ions. With the addition of Al3+ ions (10 μM) to L (10 μM), the intensity of the emission increased with an enhancement of the fluorescence quantum yield21 by about ten times (Φ = 0.708) in ethanol medium, estimated by integrating the area under the fluorescence curves with the equation:
There was almost no interference in the detection of Al3+, even in the presence of a 100× equivalent concentration of alkali and alkaline earth metal ions (Na+, K+, Mg2+, Ca2+) and 50× equivalent concentration of several transition metal ions (Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+) (Fig. S11 and S12†). Job's plot analysis (Fig. 4) revealed that the in situ formation of L yields a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 L′–Al species. The binding constant (K, 8.27 × 104 M−1) was determined from the emission intensity data (Fig. 5) using the modified Benesi–Hildebrand equation corresponding to a 1
1 L′–Al species. The binding constant (K, 8.27 × 104 M−1) was determined from the emission intensity data (Fig. 5) using the modified Benesi–Hildebrand equation corresponding to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry:22
1 stoichiometry:22
| 1/(Fx − F0) = 1/(Fmax − F0) + (1/K[C])(1/(Fmax − F0) |
 | ||
| Fig. 5 Binding constant (K) value of 8.27 × 104 M−1 for L determined from the intercept/slope of the emission plot. | ||
The measurement of the average fluorescence lifetime of L in the presence and absence of Al3+ ions in water–DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) indicates a gradual increase with increasing concentrations of Al3+ (Fig. S13†). The average lifetimes were calculated to be 7.93 ns for L alone, 6.23 ns for a mixture of L–Al3+ (1
1 v/v) indicates a gradual increase with increasing concentrations of Al3+ (Fig. S13†). The average lifetimes were calculated to be 7.93 ns for L alone, 6.23 ns for a mixture of L–Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) and 4.22 ns for L–Al3+ (1
0.5) and 4.22 ns for L–Al3+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The strong binding of Al3+ with the organic moiety, reflected in the value of the binding constant, plays a key part in the selective CHEF in the presence of Al3+ ions. The radiative rate constant kr and total non-radiative rate constant knr of L and the aluminium(III) complex were calculated using the following equations:23 τ−1 = kr + knr and kr = Φf/τ (Table S1†). The data clearly show the large increase in the ratio of kr/knr from 0.074 for L to 2.26 for L′–Al, which plays the key part in the enhancement of the fluorescence through chelation.
1). The strong binding of Al3+ with the organic moiety, reflected in the value of the binding constant, plays a key part in the selective CHEF in the presence of Al3+ ions. The radiative rate constant kr and total non-radiative rate constant knr of L and the aluminium(III) complex were calculated using the following equations:23 τ−1 = kr + knr and kr = Φf/τ (Table S1†). The data clearly show the large increase in the ratio of kr/knr from 0.074 for L to 2.26 for L′–Al, which plays the key part in the enhancement of the fluorescence through chelation.
1H-NMR titration
To strengthen our understanding of the bonding pathway of Al3+ ions with L, an 1H-NMR titration was performed by the addition of Al3+ ions to the DMSO-d6 solution of L. Significant spectral changes were observed during the addition of the Al3+ ions (Fig. S14†). After the addition of 1.0 mM Al3+ ions to the solution of 1.0 mM L, a new peak of imidazolic NH appears at δ = 8.07 ppm with the disappearance of the six-membered N–H ring. The peaks at approximately δ = 7.6, 7.58, 7.16, 7.09 and 6.7, corresponding to Hb′, Hc, Hg, Hi and Hl, are shifted to approximately δ = 7.9, 7.47, 7.38, 7.23 and 6.84, respectively. These significant spectral changes in the 1H-NMR titration emphasize the mode of chelation of L with Al3+ ions to form the L′–Al species in solution.Selectivity
The fluorescent responses of the organic moiety towards the different metal ions were investigated with 100× concentration of alkali (Na+, K+), alkaline earth (Mg2+, Ca2+) and transition metal ions (Mn2+, Ni2+, Zn2+, Cd2+, Co2+, Cu2+, Fe2+, Fe3+, Cr3+, Hg2+) and Pb2+ and Ag+ (Fig. S11 and 12†). The probe L has an extraordinary selectivity and specificity towards Al3+ ions over other competitive cations.Effect of pH
The fluorescence intensity of L was measured at various pH values by adjusting the pH using HEPES buffer in the presence and absence of Al3+ ions. In the absence of Al3+ ions, L exhibited a weak fluorescence independent of pH in the pH range 6.0–10.0 (Fig. S15†). The fluorescence intensity of the probe (L) in the presence of Al3+ ions is remarkably higher than that in the absence of Al3+ ions.Analytical figure of merit
Based on the fluorescence enhancement at 476 nm, the detection limit was calculated from the calibration graph (Fig. 6) focusing on the lower concentration region of Al3+ ions. The detection limit was estimated using the equation 3σ/S, where S is the slope of the graph and σzero is the standard deviation of seven replicate measurements of the zero level. The data from the graph indicate that this probe effectively detects Al3+ ions at very low concentrations (LOD = 1.48 nM).24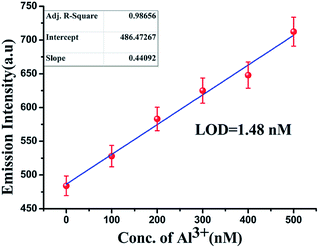 | ||
Fig. 6 Calibration graph in the nanomolar range with error bars for calculating the LOD of Al(III) by L in 100 mM HEPES buffer (DMSO–water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) at 27 °C. 9 v/v) at 27 °C. | ||
Cell imaging
The probe L was applied to the detection of MCF-7 and HeLa cells to explore its utility in biological systems. Al3+ and L were taken up by the cells of interest and images of the cells were captured by fluorescence microscopy after excitation at about 405 nm (Fig. 7 and S16†). In addition, an in vitro study showed that L has no cytotoxicity towards cells for up to 8.0 h (IC50 > 50 μM) (Fig. S17†). These results indicate that the probe has a huge potential in both in vitro and in vivo applications as an Al3+ sensor and in live cell imaging.Conclusions
A new quinazoline derivative (L) has been designed and characterized crystallographically. L behaves as a quick-response Al3+ ion-selective ratiometric chemosensor through a CHEF process in 100 mM HEPES buffer (water–DMSO, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at biological pH values. The mechanisms have been established experimentally via significant changes in the electronic, fluorimetric and 1H-NMR spectra. This bio-friendly probe is useful for the detection of intercellular Al3+ ions in HeLa and MCF-7 cells. This selective Al3+ CHEF-based ratiometric probe associated with a red shift is of better quality than previously reported red-shifting fluorescence probes in terms of the detection limit and the medium of the detection. The LOD is 1.48 nM in a green solvent.13
1 v/v) at biological pH values. The mechanisms have been established experimentally via significant changes in the electronic, fluorimetric and 1H-NMR spectra. This bio-friendly probe is useful for the detection of intercellular Al3+ ions in HeLa and MCF-7 cells. This selective Al3+ CHEF-based ratiometric probe associated with a red shift is of better quality than previously reported red-shifting fluorescence probes in terms of the detection limit and the medium of the detection. The LOD is 1.48 nM in a green solvent.13
Acknowledgements
The authors gratefully acknowledge financial assistance from the Council of Scientific and Industrial Research (CSIR), New Delhi, India. M. Mukherjee thanks the UGC, New Delhi, India for offering fellowships. The authors thank Professor B. Mukhopadhyay, IISER, Kolkata for providing the facility to record some 1H-NMR and 13C-NMR spectra and USIC, the University of Burdwan, for the single-crystal X-ray diffractometer facility under the PURSE program.Notes and references
- (a) R. P. Haugland, The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, Invitrogen, Carlsbad, CA, 10th edn, 2005 Search PubMed; (b) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed; (c) S. C. Burdette and S. J. Lippard, Coord. Chem. Rev., 2001, 216, 333 CrossRef; (d) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS PubMed.
- (a) W. S. Miller, L. Zhuang, J. Bottema, A. J. Wittebrood, P. De Smet, A. Haszler and A. Vieregge, Mater. Sci. Eng., A, 2000, 280, 37 CrossRef; (b) R. E. Doherty, Environ. Forensics, 2000, 1, 83 CrossRef CAS; (c) G. Ciardelli and N. Ranieri, Water Res., 2001, 35, 567 CrossRef CAS PubMed; (d) R. Flarend, T. Bin, D. Elmore and S. L. Hemb, Food Chem. Toxicol., 2001, 39, 163 CrossRef CAS PubMed; (e) R. A. Yokel, Food Chem. Toxicol., 2008, 46, 2261 CrossRef CAS PubMed.
- J. L. Greger, Crit. Rev. Clin. Lab. Sci., 1997, 34, 439 CrossRef CAS PubMed.
- A. Lankoff, A. Banasik, A. Duma, D. Ochniak, H. Lisowska, T. Kuszewski, S. Góźdź and A. Wojcik, Toxicol. Lett., 2006, 161, 27 CrossRef CAS PubMed.
- (a) M. Hemadi, G. Miquel, P. H. Kahn and J. M. E. H. Chahine, Biochemistry, 2003, 42, 3120 CrossRef CAS PubMed; (b) R. B. Martin, J. Savory, S. Brown, R. L. Bertholf and M. R. Wills, Clin. Chem., 1987, 33, 405 CAS; (c) A. J. Roskams and J. R. Connor, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 9024 CrossRef CAS PubMed; (d) M. Cochran, V. Chawtur, M. E. Jones and E. A. Marshall, Blood, 1991, 77, 2347 CAS; (e) S. Kim, J. Y. Noh, K. Y. Kim, J. H. Kim, H. K. Kang, S. W. Nam, S. H. Kim, S. Park, C. Kim and J. Kim, Inorg. Chem., 2012, 51, 3597 CrossRef CAS PubMed and references therein.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS.
- K. Soroka, R. S. Vithanage, D. A. Phillips, B. Walker and P. K. Dasgupta, Anal. Chem., 1987, 59, 629 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 2006 Search PubMed.
- (a) A. P. de Silva, D. B. Fox, J. M. Huxley and T. S. Moody, Coord. Chem. Rev., 2000, 205, 41 CrossRef CAS; (b) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS.
- (a) S. Sen, T. Mukherjee, B. Chattopadhyay, A. Moirangthem, A. Basu, J. Marek and P. Chattopadhyay, Analyst, 2012, 137, 3975 RSC; (b) S. Sen, T. Mukherjee, S. Sarkar, S. K. Mukhopadhyay and P. Chattopadhyay, Analyst, 2011, 136, 4839 RSC; (c) U. C. Saha, K. Dhara, B. Chattopadhyay, S. K. Mandal, S. Mondal, S. Sen, M. Mukherjee, S. V. Smaalen and P. Chattopadhyay, Org. Lett., 2011, 13, 4510 CrossRef CAS PubMed; (d) U. C. Saha, B. Chattopadhyay, K. Dhara, S. K. Mandal, S. Sarkar, A. R. Khuda-Bukhsh, M. Mukherjee, M. Helliwell and P. Chattopadhyay, Inorg. Chem., 2011, 50, 1213 CrossRef CAS PubMed; (e) K. Dhara, U. C. Saha, A. Dan, M. Manassero, S. Sarkar and P. Chattopadhyay, Chem. Commun., 2010, 46, 1754 RSC; (f) M. Mukherjee, B. Sen, S. Pal, M. S. Hundal, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, RSC Adv., 2013, 3, 19978 RSC.
- B. Sen, S. Pal, S. Lohar, M. Mukherjee, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, RSC Adv., 2014, 4, 21471 RSC and references therein.
- (a) S. M. Z. Al-Kindy, F. E. O. Suliman and A. E. Pillay, Instrum. Sci. Technol., 2006, 34, 619 CrossRef CAS; (b) J. L. Ren, J. Zhang, J. Qing Luo, X. K. Pei and Z. Xi Jiang, Analyst, 2001, 126, 698 RSC; (c) S. M. Ng and R. Narayanaswamy, Anal. Bioanal. Chem., 2006, 386, 1235 CrossRef CAS PubMed; (d) Y.-W. Wang, M.-X. Yu, Y.-H. Yu, Z.-P. Bai, Z. Shen, F.-Y. Li and X.-Z. You, Tetrahedron Lett., 2009, 50, 6169 CrossRef CAS.
- (a) D. Jeyanthi, M. Iniya, K. Krishnaveni and D. Chellappa, RSC Adv., 2013, 3, 20984 RSC; (b) J. Hatai, M. Samanta, V. S. Rama Krishna, S. Pal and S. Bandyopadhyay, RSC Adv., 2013, 3, 22572 RSC; (c) S. Goswami, S. Paul and A. Manna, RSC Adv., 2013, 3, 25079 RSC.
- (a) Z. Xu, Y. Xiao, X. Qian, J. Cui and D. Cui, Org. Lett., 2005, 7, 889 CrossRef CAS PubMed; (b) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS; (c) S. Sen, S. Sarkar, B. Chattopadhyay, A. Moirangthem, A. Basu, K. Dhara and P. Chattopadhyay, Analyst, 2012, 137, 3335 RSC.
- A. Altomare, G. Cascarano, C. Giacovazzo and A. Guagliardi, J. Appl. Crystallogr., 1993, 26, 343 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef CAS.
- J. L. McClintock and B. P. Ceresa, Invest. Ophthalmol. Visual Sci., 2010, 51, 3455 Search PubMed.
- S. Banerjee, A. Dixit, R. N. Shridharan, A. A. Karande and A. R. Chakravarty, Chem. Commun., 2014, 50, 5590–5592 RSC.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 2006 Search PubMed.
- W. H. Melhuish, J. Phys. Chem., 1961, 65, 229 CrossRef CAS.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- N. J. Turro, Modern Molecular Photochemistry, Benjamin/Cummings Publishing Co., Inc., Menlo Park, CA, 1978, p. 246 Search PubMed.
- (a) M. Mukherjee, S. Pal, B. Sen, S. Lohar, S. Banerjee, S. Banerjee and P. Chattopadhyay, RSC Adv., 2014, 4, 27665 RSC; (b) S. Pal, B. Sen, M. Mukherjee, K. Dhara, E. Zangrando, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, Analyst, 2014, 139, 1628 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1021032. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra10836a |
| This journal is © The Royal Society of Chemistry 2014 |