Evaluation of rice bran, sesame and moringa oils as feasible sources of biodiesel and the effect of blending on their physicochemical properties
M. A. Wakil*,
M. A. Kalam*,
H. H. Masjuki,
I. M. Rizwanul Fattah and
B. M. Masum
Department of Mechanical Engineering, Faculty of Engineering, University of Malaya, Kuala Lumpur 50503, Malaysia. E-mail: wakil_01@yahoo.com; kalam@um.edu.my; Fax: +60379675317
First published on 17th October 2014
Abstract
Globally, the environmental awareness is driving the research towards energy resources that are more beneficial to milieu. Biofuel is considered to be a remarkable option for that. Among the sources of biofuels, vegetable oils are the cheapest, easily available and in abundant quantity. However, some processes are needed to make vegetable oils suitable for engines because vegetable oils have certain detrimental properties. In this study, three potential feedstocks, namely, moringa, sesame and rice bran oils are critically investigated as potential sources for biodiesel production. The work was divided into several steps: firstly, the production of biodiesel from the three feedstocks; secondly, the measurement of the important physical and chemical properties of biodiesels; and finally, the development of mathematical equations with the help of polynomial curve fitting method for biodiesel–diesel and biodiesel–biodiesel blends to predict the most important properties, such as kinematic viscosity, flash point, calorific value, CFPP of the blended biodiesel. The experiment has shown that the three feedstocks can be considered to be feasible sources for biodiesel. It is seen from the experiment that biodiesel blends have notable effect on properties; for instance, the viscosity of the rice bran oil is improved to 5.1631 mm2 s−1 from 5.3657 mm2 s−1, when mixed with sesame biodiesel at a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, it is improved to 5.0921 mm2 s−1, when mixed with moringa biodiesel at a volume ratio of 3
1. Moreover, it is improved to 5.0921 mm2 s−1, when mixed with moringa biodiesel at a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, flash point and CFPP of rice bran biodiesel are also improved, when mixed with sesame or moringa biodiesel in any percentage.
1. Moreover, flash point and CFPP of rice bran biodiesel are also improved, when mixed with sesame or moringa biodiesel in any percentage.
1 Introduction
Because of the global concern about the reliability of petro-diesel and their adverse impacts on milieu, the world is converging to renewable sources that are environmentally friendly. Currently, more than 80% energy consumption is derived from fossil fuels. Fossil fuels are primarily dominating because of their high combustion efficiency, fuel adaptability and handling facilities.1 However, the significant concern about fossil fuels is the generation of toxic pollutants linked to the global warming, climate change and even certain impasse diseases.2 In turn, this phenomenon has promoted biofuels to be a prominent source of interest, including biodiesel, which is one of the prime renewable energy sources.Among the renewable feedstock, such as vegetable oils, animal fats and recycled cooking oil, vegetable oils are found to be promising feedstock for biodiesel because of their availability and large scale production ability. Globally, more than 350 oil-bearing crops are identified to be a potential source of biodiesel, which can be classified as edible and non-edible oil.3 Because of the diversity of oil-bearing crops, it is a challenge to select the potential sources for biodiesel. Therefore, myriads of research are ongoing. A number of sources from edible oil are already being used on a large scale in several countries. For instance, canola and soybean are used in USA, palm oil in Malaysia, and rapeseed oil in Europe.3–5 Sunflower, peanut, coconut, sesame oils are few other examples of edible oils. Other sources from non-edible oils, such as Jatrapha curcas, Sterculia foetida, Croton megalocarpus, Calophyllum inophyllum, Karanja, Moringa, and rice bran, have attracted considerable attention for biodiesel6,7 because the large usage of biodiesel production from edible oils has incurred serious concern on food supply.
1.1 Botanical description of rice bran, moringa and sesame feedstocks
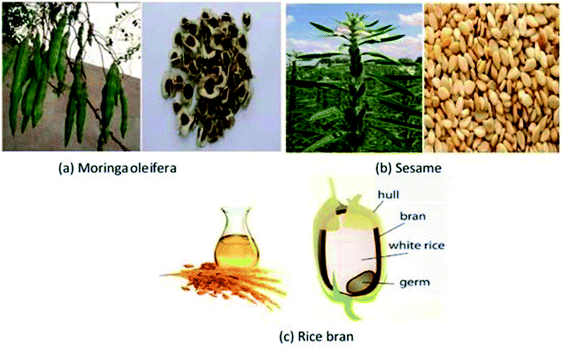
Moringa oleifera is the most widely cultivated tree species in the family of Moringaceae. It grows throughout most of the tropics and is native to sub-Himalayan tracts of north-west India, Africa, Latin America, Pakistan, Bangladesh, and Afghanistan. It is drought tolerant and can survive in arid, harsh and infertile land. The tree can range from 5–10 m in height; it can sometimes even be 15 m. The plant starts bearing pods 6–8 months after planting. The seeds are triangular in shape and contain about 35–45% oil by weight.1,8–10Rice is the seed of the monocot plants Oryza sativa (Asian rice) or Oryza glaberrima (African rice). Rice is the most important cereal cultivated in the world, which feeds more than half of the people of the world. Rice bran is the by-product of rice milling process. Because of the presence of active lipase and high free fatty acid, about 60–70% of rice bran oil production is non-edible. Rice can be practically grown anywhere, even on a steep hill. It contains about 16–32% oil by weight.11–13
Sesame (Sesamum indicum L) is an oil seed herbaceous crop of the Pedaliaceae family, primarily found in tropical and subtropical areas. It is an annual plant growing 50 to 100 cm tall with opposite leaves 4–14 cm long. The flowers are yellow, tubular with a four-lobed mouth. The flower may vary in color with some being white, blue or purple. The tree is originated in Africa, Turkey, India, China, Sudan, Burma, Tunisia, Egypt, Thailand, Mexico, Guatemala, Afghanistan, Pakistan, and Bangladesh. The oil content is about 57–63%.14,15
The pictorial view of the feedstocks is shown in Fig. 1.
1.2 Objectives of this paper
Recently, many studies have been undertaken concerning the production and properties of edible and non-edible oils.6,16–23 Some authors have discussed the production of biodiesel from Moringa oleifera,24–26 rice bran12,27,28 and sesame.14,29 However, there is no published report on the comparison of the physico-chemical properties of these feedstocks. The comparative evaluation of properties is inevitable for the maximum usage of biodiesel and optimization of biodiesel blends because research has shown that the blended biodiesel with two or more feedstocks provides better performance.30–32 Therefore, the primary objective of this study is to produce biodiesel from crude rice bran, sesame and moringa oil and critically analyze the physico-chemical properties of rice bran, sesame and moringa biodiesel, as potential sources for biodiesel. Then, the biodiesel–diesel and biodiesel–biodiesel blending is suggested to improve some of the main properties, such as kinematic viscosity, calorific value, flash point, and CFPP. In this paper the polynomial curve fitting method is suggested to predict the properties of the blended biodiesel.6,332 Materials and method
2.1 Materials
The crude moringa and sesame oils were purchased from Delhi, India. Rice bran oil was obtained from the local markets of Bangladesh. Other chemicals, such as methanol, H2SO4, KOH, Na2SO4, and qualitative filter paper of 150 mm size, were obtained from Malaysia. Biodiesel production was conducted in the scale of 1 L batch reactor.2.2 Biodiesel production
2.2.2.1 Esterification process. This process is primarily employed to reduce the acid value of the feedstock prior to the transesterification process. In this process 50% (v/v oil) methanol was reacted with refined sesame oil. 1% (v/v oil) sulfuric acid (H2SO4) was added to the preheated oil at 60 °C for 3 h with stirring at the speed of 400 rpm in a glass reactor. After completing the reaction time the product was poured in a separating funnel to separate the excess of alcohol and sulfuric acid. The upper layer containing impurities was separated; the lower layer was placed into a rotary evaporator and heated at 95 °C under vacuum condition to remove ethanol and water content.
2.2.2.2 Transesterification process. This process is same as that in Section 2.2.1.
2.3 Measurement of physico-chemical properties of crude oil and their biodiesel
The physico-chemical properties of crude oils and their biodiesel were tested according to the ASTM method. These properties include kinematic viscosity, density, calorific value, viscosity index, CFPP, cloud point, pour point, flash point and oxidation stability with some non-ASTM properties, such as absorbance, transmission and refractive index. Table 1 shows the test methods and ASTM standard.| Property | Equipment | Manufacturer | Standard method | ASTM D6751 limit | Accuracy |
|---|---|---|---|---|---|
| a n.s. ≡ not specified in ASTM test method. | |||||
| Kinematic viscosity at 40 °C | SVM 3000-automatic | Anton Paar, UK | D 445 | 1.9–6.0 | ±0.35% |
| Dynamic viscosity at 40 °C | SVM 3000-automatic | Anton Paar, UK | D7042 | n.s. | ±0.35% |
| Viscosity index | SVM 3000-automatic | Anton Paar, UK | D 2270 | ||
| Density at 40 °C | SVM 3000-automatic | Anton Paar, UK | D 7042 | n.s. | 0.0005 g cm−3 |
| Density at 15 °C | DM40 LiquiPhysics™ density meter | Mettler Toledo, Switzerland | D 4052 | ±0.1 kg m−3 | |
| Flash point | Pensky-martens flash point – automatic NPM 440 | Normalab, France | D 93 | 130 min | ±0.1 °C |
| Oxidation stability | 873 Rancimat-automatic | Metrohm, Switzerland | D 675 | 3 h min | ±0.01 h |
| Higher heating value (HHV) | C2000 basic calorimeter-automatic | IKA, UK | D 240 | n.s. | ±0.1% of reading |
| Cloud point | Cloud and pour point tester-automatic NTE 450 | Normalab, France | D 2500 | Report | ±0.1 °C |
| Pour point | Cloud and pour point tester-automatic NTE 450 | Normalab, France | D 97 | ±0.1 °C | |
| CFPP | Cold filter plugging point–automatic NTL 450 | Normalab, France | D 6371 | n.s. | |
| Acid value | G-20 rondolino automated titration system | Mettler Toledo, Switzerland | D 664 | 0.5 max | ±0.001 mg KOH per g |
2.4 Blending of biodiesel
The blending of biodiesel–diesel and biodiesel–biodiesel was done using a homogenizer at 2000 rpm. The effect of biodiesel blending ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were studied for certain properties. These include kinematic viscosity, calorific value, flash point and CFPP. For this purpose, the polynomial curve fitting method was used to predict the properties of biodiesel–diesel blends and biodieselbiodiesel blends. Mathematically, a polynomial of order k in X is expressed as follows: Y = Co + C1X + C2X2 + … + CkXk, where X is the variable as a function of available data, and Y is the predicted value.
3 were studied for certain properties. These include kinematic viscosity, calorific value, flash point and CFPP. For this purpose, the polynomial curve fitting method was used to predict the properties of biodiesel–diesel blends and biodieselbiodiesel blends. Mathematically, a polynomial of order k in X is expressed as follows: Y = Co + C1X + C2X2 + … + CkXk, where X is the variable as a function of available data, and Y is the predicted value.
3 Results and discussion
3.1 Characterization of crude oils
The properties of crude oils are presented in Table 2. The findings from the table show that both sesame and moringa oils possess almost the same kinematic viscosity of 34.087 mm2 s−1 and 32.004 mm2 s−1, respectively, while rice bran possesses the highest viscosity of 52.225 mm2 s−1. The acid value of rice bran and moringa oil is about 1 mg KOH per g (1.314 and 0.8670), while sesame oil has a considerable high acid value of 13.56 mg KOH per g. Rice bran oil possesses the highest flash point of 300.5 °C, whereas moringa and sesame oil have flash points of 263.5 °C and 280.5 °C, respectively. All the three oils have the same refractive index of about 1.47, whereas sesame possesses the highest absorbance of 0.106.| Property | Unit | CRBO | CMOO | CSO |
|---|---|---|---|---|
| a CRBO ≡ crude rice bran oil, CMOO ≡ crude moringa oleifera oil, CSO ≡ crude sesame oil. | ||||
| Kinematic viscosity at 40 °C | mm2 s−1 | 52.225 | 32.004 | 34.087 |
| Dynamic viscosity at 40 °C | mPa s | 47.364 | 29.003 | 30.905 |
| Density at 15 °C | kg m−3 | 924.3 | 923.4 | 923.6 |
| Specific gravity at 15 °C | — | 0.9251 | 0.9242 | 0.9244 |
| Density at 40 °C | kg m−3 | 906.9 | 906.3 | 906.6 |
| Kinematic viscosity at 100 °C | mm2 s−1 | 10.393 | 7.6569 | 7.6364 |
| Acid value | mg KOH per g | 1.314 | 0.8670 | 13.56 |
| Oxidation stability | h | 4.40 | 41.75 | 9.795 |
| Cloud point | °C | 0 | −7 | −3 |
| Pour point | °C | 0 | −7 | −4 |
| CFPP | °C | 16 | 9 | 44 |
| Higher heating value (HHV) | MJ kg−1 | 39.548 | 39.868 | 39.386 |
| Viscosity index | — | 192.8 | 222 | 202.9 |
| Refractive index | — | 1.4718 | 1.4728 | 1.4709 |
| Transmission | %T | 87.1 | 85.9 | 78.4 |
| Absorbance | ABS | 0.06 | 0.066 | 0.106 |
| Flash point | °C | 300.5 | 263.5 | 280.5 |
3.2 Characterization of biodiesels
The measured physical and chemical properties for rice bran, sesame and moringa biodiesel are presented in Table 3. The findings show that the kinematic viscosity of sesame and moringa biodiesel satisfy both ASTM and EN standards which are 4.3989 mm2 s−1 and 4.1264 mm2 s−1, respectively; however rice bran oil satisfies only the ASTM standard which is 5.3657 mm2 s−1. The densities of all the biodiesels are slightly higher (about 3.5%) than diesel. The calorific values of all the biodiesels are about 40 MJ kg−1, which is around 12% less than diesel. The flash points of the three feedstocks are 174.5, 208.5 and 176.5 °C, respectively, which also satisfy the ASTM and EN standards.| Properties | Unit | SME | RBME | MOME | ASTM D6751 | EN 14214 | Diesel |
|---|---|---|---|---|---|---|---|
| a n.s. ≡ not specified in ASTM test method, SME ≡ sesame methyl ester, MOME ≡ moringa oleifera methyl ester, RBME ≡ rice bran methyl ester. | |||||||
| Kinematic viscosity at 40 °C | mm2 s−1 | 4.3989 | 5.3657 | 4.1264 | 1.9–6.0 | 3.5–5.0 | 3.1818 |
| Dynamic viscosity at 40 °C | mPa s | 3.8136 | 4.6581 | 3.5781 | n.s. | n.s. | 2.6474 |
| Density at 15 °C | Kg m−3 | 884.8 | 886.9 | 885.8 | n.s. | 860–900 | 849.1 |
| Specific gravity at 15 °C | 0.8856 | 0.8877 | 0.8858 | n.s. | n.s. | 1.2723 | |
| Density at 40 °C | Kg m−3 | 866.9 | 868.1 | 867.1 | n.s. | n.s. | 832.1 |
| Kinematic viscosity at 100 °C | Mm2 s−1 | 1.7236 | 1.9609 | 1.6655 | n.s. | n.s. | 1.2723 |
| Oxidation stability | h | 1.135 | 1.61 | 12.64 | >3 | >6 | 58.51 |
| Cloud point | °C | 1 | 0 | 0 | Report | n.s. | 3 |
| Pour point | °C | 1 | −3 | −1 | n.s. | n.s. | 0 |
| CFPP | °C | −1 | 2 | −2 | n.s. | n.s. | 4 |
| Higher heating value | MJ kg−1 | 39.996 | 39.957 | 39.888 | n.s. | n.s. | 45.315 |
| Viscosity index (VI) | 229.0 | 187 | 244.9 | n.s. | n.s. | 133.2 | |
| Refractive index | 1.4540 | 1.4541 | 1.4553 | n.s. | n.s. | N/D | |
| Transmission | %T | 86.5 | 82.4 | 87.55 | n.s. | n.s. | N/D |
| Absorbance | Abs | 0.063 | 0.08 | 0.058 | n.s. | n.s. | N/D |
| Flash point | °C | 208.5 | 174.5 | 176.5 | >130 | >120 | 73.5 |
3.3 Effect of blending on physicochemical properties
| Property | Biodiesel blends | Mathematical equation | R2 | Variable |
|---|---|---|---|---|
| (a): Biodiesel–diesel blends | ||||
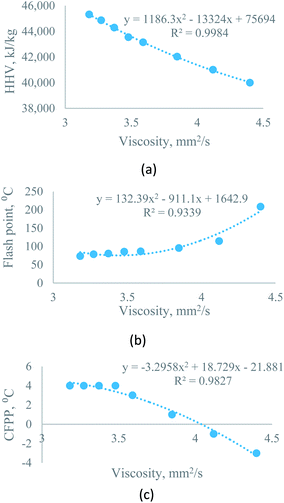
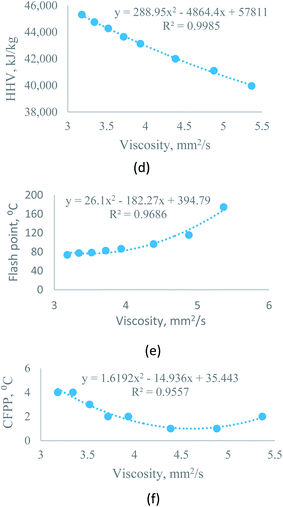
| Higher heating value vs. kinematic viscosity at 40 °C | Sesame–diesel | y = 1186.3x2 − 13324x + 75694 | R2 = 0.9984 | X = percentage of biodiesel, varies from 0 to 100 |
| Rice bran–diesel | y = 288.95x2 − 4864.4x + 57811 | R2 = 0.9985 | ||
| Moringa–diesel | y = 2135.6x2 − 21284x + 91375 | R2 = 0.9994 | ||
| Flash point vs. kinematic viscosity at 40 °C | Sesame–diesel | y = 132.39x2 − 911.1x + 1642.9 | R2 = 0.9339 | |
| Rice bran–diesel | y = 26.1x2 − 182.27x + 394.79 | R2 = 0.9686 | ||
| Moringa–diesel | y = 104.91x2 − 669.72x + 1147.7 | R2 = 0.9599 | ||
| CFPP vs. kinematic viscosity at 40 °C | Sesame–diesel | y = −3.2958x2 + 18.729x − 21.881 | R2 = 0.9827 | |
| Rice bran–diesel | y = 1.6192x2 − 14.936x + 35.443 | R2 = 0.9557 | ||
| Moringa–diesel | y = 9.5395x2 − 76.274x + 150.34 | R2 = 0.9355 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| (b): Biodiesel–biodiesel blends | ||||
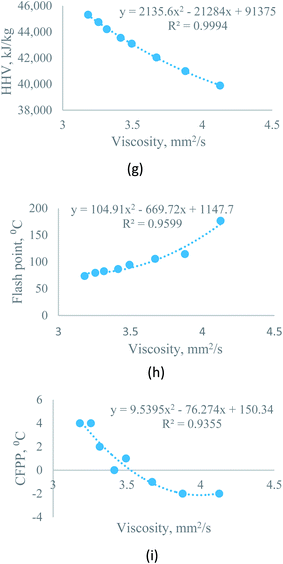
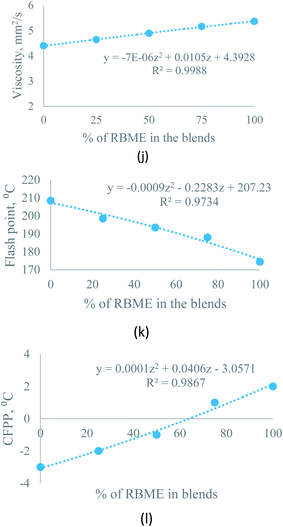
| Kinematic viscosity at 40 °C | Sesame–rice bran | Y = −7 × 10−6Z2 + 0.0105Z + 4.3928 | R2 = 0.9988 | Z = percentage of rice bran biodiesel, varies from 0 to 100 |
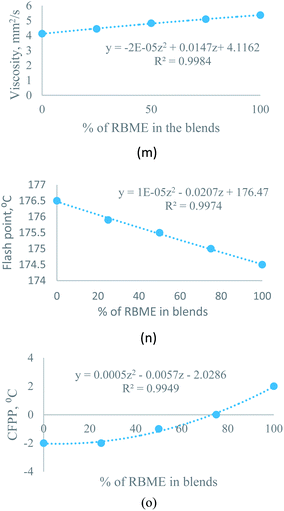
| Moringa–rice bran | Y = −2 × 10−5Z2 + 0.0147Z + 4.1162 | R2 = 0.9984 | ||
| Flash point | Sesame–rice bran | Y = −0.0009Z2 − 0.2283Z + 207.23 | R2 = 0.9734 | |
| Moringa–rice bran | Y = 1 × 10−5Z2 − 0.0207Z + 176.47 | R2 = 0.9974 | ||
| CFPP | Sesame–rice bran | Y = 0.0001Z2 + 0.0406Z − 3.0571 | R2 = 0.9867 | |
| Moringa–rice bran | Y = 0.0005Z2 − 0.0057Z − 2.0286 | R2 = 0.9949 | ||
3.3.2.1 Effect of biodiesel–biodiesel blend on kinematic viscosity. Blending has a considerable effect on kinematic viscosity. For sesame–rice bran biodiesel blend with (3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio, the viscosity of rice bran oil improved from 5.3657 mm2 s−1 to 5.1631 mm2 s−1. The equation, Y = −7 × 10−6Z2 + 0.0105Z + 4.3928, (0 ≥ Z ≤ 100) helps to predict the kinematic viscosity at any percentage of rice bran methyl ester (Z).
1) ratio, the viscosity of rice bran oil improved from 5.3657 mm2 s−1 to 5.1631 mm2 s−1. The equation, Y = −7 × 10−6Z2 + 0.0105Z + 4.3928, (0 ≥ Z ≤ 100) helps to predict the kinematic viscosity at any percentage of rice bran methyl ester (Z).Moreover, for the moringa–rice bran blend, the kinematic viscosity of rice bran also improved from 5.3657 mm2 s−1 to 5.0921 mm2 s−1. The equation, Y = −2 × 10−5Z2 + 0.0147Z + 4.1162, (0 ≥ Z ≤ 100) helps to predict the viscosity at any percentage of rice bran methyl ester (Z).
3.3.2.2 Effect of biodiesel–biodiesel blend on flash point. For both sesame–rice bran and moringa–rice bran, the flash point deflects about 3% from pure rice bran biodiesel with a ratio of 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume. Flash point improved to 175.5 °C for moringa–rice bran biodiesel and 198.5 °C for sesame–rice bran biodiesel at a ratio of 3
1 by volume. Flash point improved to 175.5 °C for moringa–rice bran biodiesel and 198.5 °C for sesame–rice bran biodiesel at a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume, where the flash point of moringa is 176.5 °C, for rice bran is 174.5 °C and for sesame biodiesels is 208.5 °C. The following equations were used to predict the flash point of sesame–rice bran and moringa–rice bran biodiesel blends for any percentage of rice bran methyl ester (0 ≥ Z ≤ 100):
1 by volume, where the flash point of moringa is 176.5 °C, for rice bran is 174.5 °C and for sesame biodiesels is 208.5 °C. The following equations were used to predict the flash point of sesame–rice bran and moringa–rice bran biodiesel blends for any percentage of rice bran methyl ester (0 ≥ Z ≤ 100):| Y = −0.0009Z2 − 0.2283Z + 207.23 | (1) |
| Y = 1 × 10−5Z2 − 0.0207Z + 176.47 | (2) |
3.3.2.3 Effect of biodiesel–biodiesel blend on cold filter plugging point. The cold filter plugging point (CFPP) can be easily predicted for sesame–rice bran and moringa–rice bran biodiesel blend using eqn (3) and (4) with condition (0 ≥ Z ≤ 100). CFPP of sesame–rice bran blended biodiesel improved to −1 °C at 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio by volume, where CFPP of rice bran is 2 °C.
1 ratio by volume, where CFPP of rice bran is 2 °C.| Y = 0.0001Z2 + 0.0406Z − 3.0571 | (3) |
| Y = 0.0005Z2 − 0.0057Z − 2.0286 | (4) |
3.4 Validation of mathematical equations for biodiesel blends
The equation developed using the polynomial curve fitting method for various biodiesel blend percentages are validated with the experimental data shown in Table 5. The variation of data is calculated using eqn (5).34 Viscosity variation with experimental data for each blend is found below 0.5% and not more than 5.5%, when it is used for flash point calculation. The variation of CFPP is considerably larger, about 20%.
 | (5) |
| Blend | Property | Biodiesel blend | Experimental data | Data from equation | Variation % |
|---|---|---|---|---|---|
| Rice bran + diesel | Higher heating value | B20 | 44.206 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279.29 279.29 |
0.14 |
| B60 | 42.042 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019.61 019.61 |
|||
| Flash point | B20 | 82.5 | 80.486 | 3.79 | |
| B60 | 105.5 | 102.98 | |||
| CFPP | B20 | 2 | 2.469 | 9.27 | |
| B60 | −1 | −0.8839 | |||
| Sesame + diesel | Higher heating value | B20 | 44.291 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256.29 256.29 |
0.13 |
| B60 | 42.026 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 989.75 989.75 |
|||
| Flash point | B20 | 80.5 | 76 | 5.45 | |
| B60 | 95.5 | 97.278 | |||
| CFPP | B20 | 4 | 3.8 | 19.24 | |
| B60 | −1 | −1.3882 | |||
| Moringa + diesel | Higher heating value | B20 | 44.278 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 263.7 263.7 |
0.10 |
| B60 | 41.995 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031.71 031.71 |
|||
| Flash point | B20 | 78.5 | 76.59 | 3.99 | |
| B60 | 96.5 | 97.49 | |||
| CFPP | B20 | 3 | 2.9248 | 19.30 | |
| B60 | 1 | 1.0784 | |||
| Sesame + rice bran | Kinematic viscosity | B25 | 4.6421 | 4.65 | 0.23 |
| B50 | 4.8933 | 4.9 | |||
| Flash point | B25 | 198.5 | 200.935 | 0.94 | |
| B50 | 193.5 | 193.56 | |||
| CFPP | B25 | −2 | −1.9796 | 22.76 | |
| B50 | −1 | −0.7771 | |||
| Moringa + rice bran | Kinematic viscosity | B25 | 4.4417 | 4.471 | 0.46 |
| B50 | 4.8216 | 4.8 | |||
| Flash point | B25 | 175.9 | 175.9587 | 0.03 | |
| B50 | 175.5 | 175.45 | |||
| CFPP | B25 | −2 | −1.8586 | 16.36 | |
| B50 | −1 | −1.0636 |
4 Conclusion
Biodiesel is one of the best potential alternatives of petro-diesel because it has profitable benefit, though it has some disadvantages, such as high kinematic viscosity, density, low volatility and heating value. This article deals with the production and characterization of biodiesel from three potential feedstocks, viz., rice bran, moringa and sesame oil. Moreover, the experimental validation of physicochemical properties of biodiesel–diesel and biodiesel–biodiesel blends is studied. By applying the curve fitting method, equations are developed for predicting important properties, which show very close-fit to the experimental data. This will help future research, such as the optimization of blending percentage, engine combustion and performance and emission analysis.Acknowledgements
The authors would like to acknowledge the University of Malaya for financial support through the High Impact Research grant titled: Development of Alternative and Renewable Energy Career (DAREC); grant number UM.C/HIR/MOHE/ENG/60.References
- M. Mofijur, H. H. Masjuki, M. A. Kalam, A. E. Atabani, I. M. R. Fattah and H. M. Mobarak, Ind. Crops Prod., 2014, 53, 78–84 CrossRef CAS PubMed.
- E. F. Aransiola, T. V. Ojumu, O. O. Oyekola, T. F. Madzimbamuto and D. I. O. Ikhu-Omoregbe, Biomass Bioenergy, 2014, 276–297 CrossRef CAS PubMed.
- A. S. Silitonga, H. H. Masjuki, T. M. I. Mahlia, H. C. Ong, W. T. Chong and M. H. Boosroh, Renewable Sustainable Energy Rev., 2013, 22, 346–360 CrossRef CAS PubMed.
- A. E. Atabani, A. S. Silitonga, H. C. Ong, T. M. I. Mahlia, H. H. Masjuki, I. A. Badruddin and H. Fayaz, Renewable Sustainable Energy Rev., 2013, 18, 211–245 CrossRef CAS PubMed.
- P. Saxena, S. Jawale and M. H. Joshipura, Procedia Eng., 2013, 51, 395–402 CrossRef CAS PubMed.
- A. E. Atabani, T. M. I. Mahlia, H. H. Masjuki, I. A. Badruddin, H. W. Yussof, W. T. Chong and K. T. Lee, Energy, 2013, 58, 296–304 CrossRef CAS PubMed.
- N. El Boulifi, A. Bouaid, M. Martinez and J. Aracil, Renewable Energy, 2013, 53, 141–147 CrossRef CAS PubMed.
- O. Kibazohi and R. S. Sangwan, Biomass Bioenergy, 2011, 35, 1352–1356 CrossRef CAS PubMed.
- S. Zhao and D. Zhang, Sep. Purif. Technol., 2013, 118, 497–502 CrossRef CAS PubMed.
- R. Ayerza, Ind. Crops Prod., 2012, 36, 70–73 CrossRef PubMed.
- N. Kumar, Varun and S. R. Chauhan, Renewable Sustainable Energy Rev., 2013, 21, 633–658 CrossRef CAS PubMed.
- L. Lin, D. Ying, S. Chaitep and S. Vittayapadung, Appl. Energy, 2009, 86, 681–688 CrossRef CAS PubMed.
- L. Danielski, C. Zetzl, H. Hense and G. Brunner, J. Supercrit. Fluids, 2005, 34, 133–141 CrossRef CAS PubMed.
- A. Saydut, M. Z. Duz, C. Kaya, A. B. Kafadar and C. Hamamci, Bioresour. Technol., 2008, 99, 6656–6660 CrossRef CAS PubMed.
- N. E. Mohamed and M. M. Wakwak, J. Radiat. Res. Appl. Sci., 2014, 101–109 CrossRef CAS PubMed.
- Q. You, X. Yin, Y. Zhao and Y. Zhang, Bioresour. Technol., 2013, 148, 202–207 CrossRef CAS PubMed.
- Widayat, A. D. K. Wibowo and Hadiyanto, Energy Procedia, 2013, 32, 64–73 CrossRef CAS PubMed.
- G. Kafuku, M. K. Lam, J. Kansedo, K. T. Lee and M. Mbarawa, Bioresour. Technol., 2010, 101, 7000–7004 CrossRef CAS PubMed.
- N. A. Ludin, M. A. M. Bakri, N. Kamaruddin, K. Sopian, M. S. Deraman, N. H. Hamid, N. Asim and M. Y. Othman, J. Cleaner Prod., 2014, 65, 9–15 CrossRef PubMed.
- M. A. K. I. M. Rizwanul Fattah, H. H. Masjuki and M. A. Wakil, RSC Adv., 2014, 17787–17796 RSC.
- Z. J. Jie Xu, L. Lia and T. Fang*, RSC Adv., 2014, 23447–23455 Search PubMed.
- X. Z. a. D. L. Morikawa Yuichi, RSC Adv., 2014, 37878–37888 RSC.
- S. L. Danlin Zeng, W. Gong, H. Chena and G. Wanga, RSC Adv., 2014, 39, 20535–20539 RSC.
- J. P. V. da Silva, T. M. Serra, M. Gossmann, C. R. Wolf, M. R. Meneghetti and S. M. P. Meneghetti, Biomass Bioenergy, 2010, 34, 1527–1530 CrossRef CAS PubMed.
- G. Kafuku and M. Mbarawa, Appl. Energy, 2010, 87, 2561–2565 CrossRef CAS PubMed.
- R. Ayerza, Ind. Crops Prod., 2011, 33, 389–394 CrossRef CAS PubMed.
- Y. Zhang, W.-T. Wong and K.-F. Yung, Bioresour. Technol., 2013, 147, 59–64 CrossRef CAS PubMed.
- S. Sinha, A. K. Agarwal and S. Garg, Energy Convers. Manage., 2008, 49, 1248–1257 CrossRef CAS PubMed.
- N. R. Banapurmath, P. G. Tewari and R. S. Hosmath, Renewable Energy, 2008, 33, 1982–1988 CrossRef CAS PubMed.
- D. S. Serqueira, D. M. Fernandes, R. R. Cunha, A. L. Squissato, D. Q. Santos, E. M. Richter and R. A. A. Munoz, Fuel, 2014, 118, 16–20 CrossRef CAS PubMed.
- A. Sarin, R. Arora, N. P. Singh, R. Sarin and R. K. Malhotra, Energy, 2010, 35, 3449–3453 CrossRef CAS PubMed.
- H. H. M. M. I. Arbab, M. Varman, M. A. Kalam, H. Sajjada and S. Imtenana, RSC Adv., 2014, 37122–37129 RSC.
- A. E. Atabani, M. Mofijur, H. H. Masjuki, I. A. Badruddin, M. A. Kalam and W. T. Chong, Ind. Crops Prod., 2014, 60, 130–137 CrossRef CAS PubMed.
- P. Benjumea, J. Agudelo and A. Agudelo, Fuel, 2008, 87, 2069–2075 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |