An overview: synthesis of thin films/membranes of metal organic frameworks and its gas separation performances
Abstract
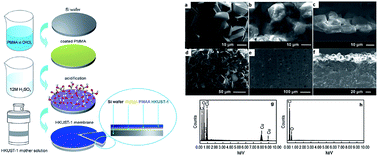
Metal organic frameworks (MOFs), an emerging class of porous solid materials, have developed into a constructive research field with intense research interests mainly in the field of materials science and chemistry. Now in the early stages of its development, research progress in MOFs membranes has exhibited promising results despite several challenges associated with its fabrications. In this review, we introduced the methods in the fabrication of MOF membranes (in situ and secondary growth) and challenges associated with MOFs membrane fabrication such as poor interaction with its substrates, moisture instability and easily induced macroscopic cracks, followed by strategies of researchers adopted in overcoming these difficulties. Moreover, the gas separation performances of these MOFs membranes were discussed and compared.

 Please wait while we load your content...
Please wait while we load your content...