Deprotection of oximes, imines, and azines to the corresponding carbonyls using Cu-nanoparticles on cellulose template as green reusable catalyst†
Diganta Baruaha,
Ujwal Pratim Saikiaa,
Pallab Pahari*a,
Dipak Kumar Duttab and
Dilip Konwar*a
aSynthetic Organic Chemistry Division, CSIR-North East Institute of Science and Technology, 785006, Jorhat, Assam, India. E-mail: ppahari@gmail.com; dkonwar@yahoo.co.uk
bMaterials Science Division, CSIR-North East Institute of Science and Technology, 785006, Jorhat, Assam, India
First published on 23rd October 2014
Abstract
The deprotection of wide varieties of oximes, imines, and azines to their corresponding carbonyls has been achieved using Cu-nanoparticles on a cellulose template as a reusable catalyst. The reactions were carried out at 80–100 °C using microwave irradiation in water under neutral condition. The catalyst can be reused for several cycles with good to excellent yield.
The protection–deprotection of functional group is a very common and widely used technique in organic synthesis.1 Generally, sensitive functional groups need to be protected to have a better control over the chemistry during a multistep reaction sequence. In many cases, the protecting groups also show activating and directing properties.2 Oximes, imines, and azines are frequently used as protecting groups for carbonyls because they provide relatively stable compounds compared to others. These compounds can also be prepared from the sources other than carbonyls, such as alcohols,3 amines,4 nitriles,5 nitroalkanes,6 and azaarenes,7 which sometimes provide newer synthetic avenues for the selective insertion of aldehydes and ketones. There are numerous methodologies reported for the deprotection of oximes and related compounds.1,8 The classical method for the cleavage of oximes to carbonyls is acid hydrolysis,9 although many other reagents, such as pyridinium chlorochromate (PCC) and related Cr(VI) compounds,10 KMnO4/alumina,11 trimethylphosphine and 2,2′-dipyridyl diselenide,12 vanadomolybdophosphate (H6PMo9V3O40),13 mixed metal oxide CeO2–ZrO2,14 FeCl3/TEMPO,15 iron(III) porphyrin complex,16 gaseous NO2,17 Cu(I) chloride/kieselghur,18 CuCl2, 2H2O,19 mixed (Fe2+ and Cu2+) double metal hexacyanocobaltates,20 platinum(II) terpyridyl acetylide complex/O2/hν,21 TiO2/mesoporous silica,22 cobalt(II) phthalocyanine/ionic liquid,23 Au/CeO2,24 I2/water,25 2-nitro-4,5-dichloropyridazin-3(2H)-one,26 and NaNO2,27 have been also reported. Most of these methods are considerably efficient and produce good yields; however, they suffer from one or more serious drawbacks (Table 1), such as requiring the use of strong Lewis and Bronsted acids,9 use of costly and hazardous metal/ligands (i.e., Mn, Cr, Ti, Au, and Pt),10–16,20–24 use of toxic solvents, difficulty in catalyst/product separation, problems associated with waste disposal, necessity of robust reaction conditions, generation of over-oxidized products, and long reaction time. Moreover, many of the catalysts are homogeneous, and thus cannot be recycled.25–27 Therefore, the development of an efficient and re-usable heterogeneous catalyst, which can deprotect oximes, imines, and azines in water under neutral conditions can be considered to be highly useful.
| Sl. no. | Reagents | Advantage/disadvantage |
|---|---|---|
| 1 | Acid hydrolysis | Use of strong Bronsted or Lewis acid |
| 2 | Pyridinium chlorochromate (PCC) and related Cr(VI) compounds10 | Use of toxic chromium(VI) reagents |
| 3 | KMnO4/alumina | Accumulation of manganese dioxide and lack of catalyst recyclability |
| 4 | Trimethylphosphine and 2,2′-dipyridyl diselenide12 | Use of toxic and costly reagents and metals |
| 5 | FeCl3/TEMPO15 | Use of costly reagents and lack of catalyst recyclability |
| 6 | Gaseous NO2 (ref. 17) | Use of corrosive and extreme reaction conditions |
| 7 | CuCl2, 2H2O19 | Lack of catalyst recyclability |
| 8 | Platinum(II) terpyridyl acetylide complex/O2/hν (ref. 22) | Use of costly reagents |
| 9 | Au/CeO2 (ref. 24) | Use of costly reagents |
| 10 | I2/water25 | Lack of catalyst recyclability |
| 11 | 2-Nitro-4,5-dichloropyridazin-3(2H)-one26 | Lack of catalyst recyclability |
| 12 | NaNO2 (ref. 27) | Lack of catalyst recyclability |
| 13 | Cellulose supported Cu-nanoparticles | Cost-effective, re-usable heterogeneous catalyst. Works in comparatively milder reaction conditions under microwave heating |
The stabilization of metal nanoparticles using biopolymers, such as cellulose, in place of synthetic polymers is currently attracting increasing importance because of the possible environmental benefits.28 Cellulose consists of anhydroglucose units joined by an oxygen linkage (β-1-4 glucosidic bond), which forms a molecular chain. As a whole, the structure creates microfibrils with widths of up to 30 nm that are three dimensionally connected to each other.29 Because of strong electrostatic ion dipole interaction, the metal nanoparticles are stabilized in these microfibrils. Moreover, depending on the different porosities of the microfibril templates, the shape and size of the nanoparticles are varied.30 Although various properties of cellulose-supported metal nanoparticles have been studied, their potential applications as renewable heterogeneous catalyst have not yet been widely explored. In this context, herein, we report the application of cellulose-supported Cu-nanoparticles as a renewable green catalyst for the efficient deprotection of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds of oximes, imines and azines to their corresponding carbonyls.
N bonds of oximes, imines and azines to their corresponding carbonyls.
In continuation of our ongoing work on nanomaterials,31 we were interested in studying the catalytic properties of cellulose supported Cu-nanoparticles. Usually, cellulose-supported Cu-nanoparticles are prepared by reducing Cu(II) on a cellulose surface using sodium borohydride.32 We have prepared the nanoparticles using hydrazine hydrate/NaOH as a reducing agent in place of NaBH4. It was found that the reduction of Cu(II) acetate on cellulose using either of the two processes provided a stable black colored Cu-cellulose mixture. An initial powder X-ray diffraction study (XRD) showed that in combination with four cellulose peaks at 2θ values of 14.8°, 16.4°, 20.4°, and 22.9°, corresponding to the Bragg's planes (101), (101′), (021), and (002), there were four additional peaks at 2θ values of 34.4°, 36.3°, 61.4° and 74.1° corresponding to the (002), (111), (220) and (311) planes, representing Cu2O, and another two distinguished peaks at 2θ values of 43.2° and 50.4° corresponding to the (111) and (200) planes, representing Cu(0) (Fig. 1). No peaks corresponding to Cu(II) or any other impurity were observed, which confirmed the existence of only Cu(0) and Cu(I). The TEM study showed that the size of the metal particles were in the range of 2–10 nm, with an average size of 6 nm (Fig. 2). The high resolution TEM identified the reticular lattice planes inside the nanoparticles, indicating their crystalline nature.
 | ||
| Fig. 2 (A) TEM and HRTEM (inset) images of the Cu(0)/Cu2O nanoparticles on a cellulose template. (B) Location of selected area electron diffraction (SAED) pattern. | ||
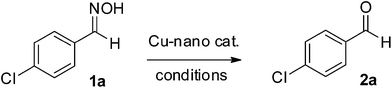
To find out the catalytic properties of the prepared Cu-nanoparticles, the deoximation of 4-chlorobenzaldehyde oxime (1a) was studied. Refluxing a mixture of 1a and the Cu-nanocatalyst in acetonitrile provided the corresponding aldehyde 2a, but the yield was low and the reaction time was also very long (Entry 1, Table 2). Influenced by the reports of a dramatic reaction rate acceleration and yield improvement by microwave irradiation, it was decided to carry out the reaction under microwave irradiation.33 Therefore, the same reaction was repeated under microwave irradiation in a sealed vial, which provided a reasonably good yield of the product 2a in a considerably less time (Entry 3, Table 2). The requirement of the metal nanoparticles in the reaction was established by performing two blank reactions, where the substrate was heated in the absence of catalyst (Entry 2 and 4, Table 2). Both the reactions failed to provide any isolable product. It was observed that only a catalytic amount of nanoparticles was sufficient to carry out the reaction, and also that water could effectively be used as solvent. Under the optimized reaction conditions, a mixture of 1a and a catalytic amount of Cu-nanoparticles was heated under microwave irradiation in a closed vial at 80 °C for 5 minutes (Entry 7, Table 2). To widen the scope of the reaction, different aromatic and heterocyclic oximes were tested (Table 3). In all the cases, the reaction smoothly proceeded to produce the corresponding carbonyls. Although the rate of the reactions did not significantly change, the yields somehow varied with the substituents present in the compounds. The electron-donating substituents, such as –OH, –NMe2, and NH2, led to a comparatively lower yield of the products. The catalyst was equally effective for both, aldoximes and ketoximes. All the products were well characterized by the comparison of their physical characteristics (TLC Rf value and melting point) and spectral data (IR and NMR) with those of the authentic samples.
| Entry | Catalyst loadinga | Heating condition | Solvent | Temperature | Time | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reactions are carried out using 1 mmol substrate.b Yields refer to isolated yield. | ||||||
| 1 | Stoichiometric | Normal heating | CH3CN | 100 °C | 48 h | 40 |
| 2 | No catalyst | Normal heating | CH3CN | 100 °C | 48 h | 0 |
| 3 | Stoichiometric | Microwave heating | CH3CN | 140 °C | 5 min | 89 |
| 4 | No catalyst | Microwave heating | CH3CN | 140 °C | 10 min | 0 |
| 5 | 20 mol% | Microwave heating | CH3CN | 140 °C | 5 min | 89 |
| 6 | 20 mol% | Microwave heating | CH3CN | 80 °C | 5 min | 89 |
| 7 | 20 mol% | Microwave heating | H2O | 80 °C | 5 min | 89 |
| Entries | Oximes | Temperature (°C)/time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: mixture of oxime (1 mmol), Cu-nano catalyst (20 mol%), and H2O (5 mL) was heated in a sealed microwave vial at a given temperature for the stipulated time.b Yields refer to the isolated yield of the carbonyls. | |||
| 1 | 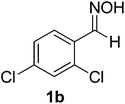 |
80/5 | 87 |
| 2 |  |
80/5 | 88 |
| 3 | 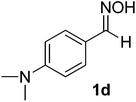 |
100/8 | 75 |
| 4 |  |
100/8 | 77 |
| 5 | 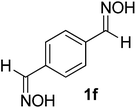 |
100/8 | 85 |
| 6 | 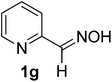 |
80/5 | 79 |
| 7 | 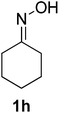 |
100/5 | 70 |
| 8 | 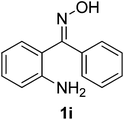 |
100/8 | 79 |
After the successful experiment with oximes, the deprotection of imines and azines were studied. It was observed that the catalyst was also able to efficiently cleave imines and azines to their corresponding amines and carbonyls (Table 4). A number of different imines and azines were deprotected using the standardized reaction condition. In this case, the yield also varied with the nature of the substituents. For all the reactions, the formation of over-oxidized products, such as acid or acid decarboxylated compounds, were not observed. Different sensitive functional groups, such as hydroxyl and alkene, were well tolerated in the reaction condition. All the products were well characterized by co-TLC expression and by comparison of the melting point, IR, and NMR data with the corresponding carbonyls. To check the recyclability, the catalyst was filtered after the reaction and reused up to 4 times without a major loss of catalytic activity (Fig. 3).
| Entries | Imines and azines | Temperature (°C), time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: Mixture of imines/azines (1 mmol), Cu-nanocatalyst (20 mol%), and H2O (5 mL) was heated in a sealed microwave vial at a given temperature for the stipulated time.b Yields refer to the isolated yield of the carbonyls. | |||
| 1 | 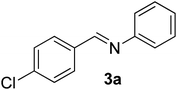 |
80/5 | 88 |
| 2 | 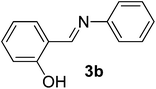 |
100/8 | 74 |
| 3 | 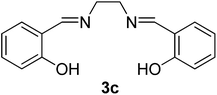 |
100/8 | 72 |
| 4 | 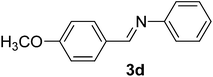 |
100/5 | 75 |
| 5 | 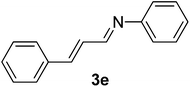 |
100/8 | 82 |
| 6 | 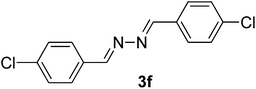 |
80/8 | 87 |
| 7 | 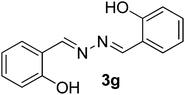 |
100/7 | 76 |
| 8 |  |
100/8 | 83 |
There are two different mechanisms reported for the Cu-catalyzed deprotection of oximes, imines, and azines.18,19 While oxidative deoximation is proposed for Cu(I) promoted reactions,18 in the case of Cu(II),19 it is only the co-ordination with nitrogen that increases the polarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and leads to nucleophilic attack by water. In the current catalytic system, there is a mixture of Cu(0) and Cu(I). To test the possibility of oxidative deoximation, an experiment was carried out under a strict inert atmosphere. It was observed that the reaction was equally effective under the inert atmosphere as well. In this situation, the co-ordination of Cu(0)/Cu(I) with the nitrogen of C
N and leads to nucleophilic attack by water. In the current catalytic system, there is a mixture of Cu(0) and Cu(I). To test the possibility of oxidative deoximation, an experiment was carried out under a strict inert atmosphere. It was observed that the reaction was equally effective under the inert atmosphere as well. In this situation, the co-ordination of Cu(0)/Cu(I) with the nitrogen of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N is the most possible mechanism for the deprotection of the oximes, imines, and azines. An alternative mechanism may also be possible for oximes only. It starts with the oxidative insertion of Cu(0) into the oximes to produce an intermediate A (Scheme 1). Subsequently, the nucleophilic attack of water cleaves the C
N is the most possible mechanism for the deprotection of the oximes, imines, and azines. An alternative mechanism may also be possible for oximes only. It starts with the oxidative insertion of Cu(0) into the oximes to produce an intermediate A (Scheme 1). Subsequently, the nucleophilic attack of water cleaves the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N via intermediate B, thus releasing the carbonyls.
N via intermediate B, thus releasing the carbonyls.
In conclusion, cellulose-supported Cu-nanoparticles were shown to be an effective heterogeneous catalyst for deprotecting oximes, imines, and azines to their corresponding carbonyls and amines. The superiority of the catalytic system over the other existing reactions can be described by few salient features, such as neutral reaction condition, ability to perform the reaction in water, very short reaction time, no use of any expensive reagents or ligands, recyclability of the catalyst, and clean reaction profile. Studies for the application of the catalytic system towards more complex substrates and different reactions are being performed.
The work is financially supported by CSIR, New Delhi, through the grant CSC0125 and MLP3000/03. The authors thank the Analytical and Material Science Division of CSIR-NEIST for recording the NMR spectra and XRD data, respectively. The TEM analysis was carried out in the analytical facility, North Eastern Hill University, Shillong, Meghalaya, India.
References
- (a) T. W. Greene, in Protective Groups in Organic Synthesis, John Wiley & Sons, New York, 1981 Search PubMed; (b) S. R. Sandler and W. Karo, in Organic Functional Group Preparations, Academic Press, London, 1989, p. 430 Search PubMed.
- (a) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (b) J. K. Whitesell and M. A. Whitesell, Synthesis, 1983, 54 Search PubMed.
- D. H. R. Barton and J. M. Beaton, J. Am. Chem. Soc., 1961, 83, 4076 CrossRef CAS.
- (a) K. Kahr and C. Berther, Chem. Ber., 1960, 93, 132 CrossRef CAS; (b) S. Wang, J. Nie, Y. Zheng and J.-A. Ma, Org. Lett., 2014, 16, 1606 CrossRef CAS PubMed.
- O. Touster, Org. React., 1953, 7, 327 Search PubMed.
- (a) C. Czekelius and E. M. Carreira, Angew. Chem., Int. Ed., 2005, 44, 612 CrossRef CAS PubMed; (b) A. Corma, P. Serna and H. García, J. Am. Chem. Soc., 2007, 129, 6358 CrossRef CAS PubMed.
- X. Gao, F. Zhang, G. Deng and L. Yang, Org. Lett., 2014, 16, 3664 CrossRef CAS PubMed.
- (a) A. Corsaro, U. Chiacchio and V. Pistarà, Synthesis, 2001, 1903 CrossRef CAS PubMed; (b) A. R. Hajipour, S. Khoee and A. E. Ruoho, Org. Prep. Proced. Int., 2003, 35, 527 CrossRef CAS.
- (a) E. J. Corey, P. B. Hopkins, S. Kim, S. Yoo, K. P. Nambiar and J. R. Falck, J. Am. Chem. Soc., 1979, 101, 7131 CrossRef CAS; (b) E. B. Hershberg, J. Org. Chem., 1948, 13, 542 CrossRef CAS.
- (a) J. R. Maloney, R. E. Lyle, J. E. Scovedra and G. G. Lyle, Synthesis, 1978, 212 CrossRef CAS; (b) A. R. Mahjoub, S. Ghammami and M. Z. Kassaee, Tetrahedron Lett., 2003, 44, 4555 CrossRef CAS; (c) S. K. De, Synth. Commun., 2004, 34, 2751 CrossRef CAS PubMed; (d) M. H. Ali, S. Greene, C. J. Wiggin and S. Khan, Synth. Commun., 2006, 36, 1761 CrossRef CAS.
- G. H. Imanzadeh, A. R. Hajipour and S. E. Mallakpour, Synth. Commun., 2003, 33, 735 CrossRef CAS PubMed.
- M. Martin, G. Martinez, F. Urpi and J. Vilarrase, Tetrahedron Lett., 2004, 45, 5559 CrossRef CAS PubMed.
- M. M. Heravi, L. Ranjbar, F. Derikvand, H. A. Oskooie and F. F. Bamobarram, J. Mol. Catal. A: Chem., 2007, 265, 186 CrossRef CAS PubMed.
- S. S. Deshpande, S. U. Sonavane and R. V. Jayaram, Catal. Commun., 2008, 9, 639 CrossRef CAS PubMed.
- G. Zhang, X. Wen, Y. Wang, X. Han, Y. Luan, L. Zheng, C. Ding and X. Cao, RSC Adv., 2013, 3, 22918 RSC.
- C. C. Y. Wang, D. M. Ho and J. T. Groves, J. Am. Chem. Soc., 1999, 121, 12094 CrossRef CAS.
- J. Mokhtari, M. R. Naimi-Jamal, H. Hamzeali, M. G. Dekamin and G. Kaupp, ChemSusChem, 2009, 2, 248 CrossRef CAS PubMed.
- M. M. Hashemi and Y. A. Beni, Synth. Commun., 2001, 31, 295 CrossRef CAS PubMed.
- N. Quan, X.-X. Shi, L.-D. Nie, J. Dong and R.-H. Zhu, Synlett, 2011, 1028 CAS.
- A. García-Ortiz, A. Grirrane, E. Reguera and H. García, J. Catal., 2014, 311, 386–392 CrossRef PubMed.
- Y. Yang, D. Zhang, L. Z. Wu, B. Chen, L. P. Zhang and C. H. Tung, J. Org. Chem., 2004, 69, 4788 CrossRef CAS PubMed.
- S. Abedi, B. Karimi, F. Kazemi, F. Bostina and H. Valib, Org. Biomol. Chem., 2013, 11, 416 CAS.
- A. Shaabani and E. Farhangi, Appl. Catal., A, 2009, 371, 148 CrossRef CAS PubMed.
- A. Grirrane, A. Corma and H. Garcia, J. Catal., 2009, 268, 350 CrossRef CAS PubMed.
- P. Gogoi, P. Hazarika and D. Konwar, J. Org. Chem., 2005, 70, 1934 CrossRef CAS PubMed.
- B. R. Kim, H. G. Lee, E. J. Kim, S. G. Lee and Y. J. Yoon, J. Org. Chem., 2010, 75, 484 CrossRef CAS PubMed.
- G. Zhang, X. Wen, Y. Wang, W. Mo and C. Ding, J. Org. Chem., 2011, 76, 4665 CrossRef CAS PubMed.
- (a) J. Cai, S. Kimura, M. Wada and S. Kuga, Biomacromolecules, 2009, 10, 87 CrossRef CAS PubMed; (b) D. Klemm, F. Kramer, S. Moritz, T. Lindstrom, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438 CrossRef CAS PubMed.
- (a) R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC; (b) Y. Habibi, Chem. Soc. Rev., 2014, 43, 1519 RSC.
- U. Vainio, K. Pirkkalainen, K. Kisko, G. Goerigk, N. E. Kotelnikova and R. Serimaa, Eur. Phys. J. D, 2007, 42, 93 CrossRef CAS.
- P. Hazarika, P. Gogoi, S. Hatibaruah and D. Konwar, Green Chem. Lett. Rev., 2011, 4, 327 CrossRef CAS.
- R. J. B. Pinto, M. C. Neves, C. P. Neto and T. Trindade, in Nanocomposites – New Trends and Developments, ed. F. Ebrahimi, ch. 4, 2012, ISBN 978-953-51-0762-0 Search PubMed.
- C. O. Kappe, D. Dallinger and S. S. Murphree, Practical Microwave Synthesis for Organic Chemists-Strategies, Instruments, And Protocols, Wiley-VCH, Verlag GmbH & Co. KGaA, Weinheim, 1st edn, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization of all compounds, and copies of NMR spectra. See DOI: 10.1039/c4ra08803d |
| This journal is © The Royal Society of Chemistry 2014 |