Up-converted fluorescence emission under linear common spectrofluorometer from PAMAM pyridine derivatives and with QDs nanoparticles†
Abstract
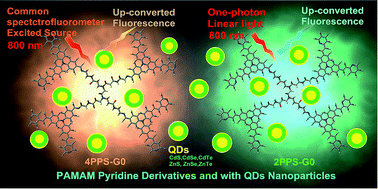
Organic dye two-photon absorption-induced frequency up-converted fluorescence (UCF) excited using a laser has been used in biological imaging and optical physics. The laser requires higher-level equipment that limits the application of UCF dyes. The experimental observation that the poly-amidoamine (PAMAM) pyridine derivatives 2PPS-G0 and 4PPS-G0 and those mixed with quantum dots (QDs) nanoparticles realized UCF using a common spectrofluorometer. The low-energy linear one-photon light source can also excite some organic molecules or QDs to give UCF emission, which replaces the need of a laser. The UCF related mechanism: second harmonic generation; two-photon absorption induced, and linear excited-states shifts (LESS) mechanisms were discussed to explain the linear one-photon light source excited UCF.

 Please wait while we load your content...
Please wait while we load your content...