Tunable dielectric constant of polyimide–barium titanate nanocomposite materials as the gate dielectrics for organic thin film transistor applications†
Abstract
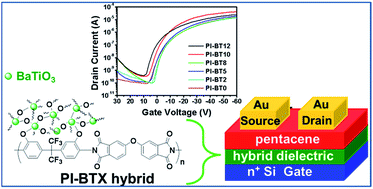
We report on a systematic study of hydroxyl-containing polyimide (PI)–BaTiO3 (BT) nanoparticle (NP) nanocomposite dielectric materials, to determine the effects of BT NPs loadings (X) for X = 0, 2, 5, 8, 10, and 12 wt%, on p-type pentacene organic thin film transistors (OTFTs). A condensation reaction to produce well-dispersed BT NPs within the PI matrix was followed by spin-coating to form a dielectric thin film directly on a silicon substrate. The thermal, optical, surface, dielectric, and electrical properties of the PI–BPX hybrid dielectric composite correlated to BT content for each sample. The hybrid dielectric composites exhibit tunable insulating properties, including high dielectric constant values in the range 5.2–11.3, high capacitances from 3.1 to 27.9 nF cm−2 for a film thickness of approximately 350 nm, and low leakage current densities in the range of 1.85 × 10−7 to 2.76 × 10−6 A cm−2 at 2 MV cm−1. Bottom-gate top-contact OTFTs fabricated using various PI–BTX hybrid dielectrics, exhibit low threshold voltages of −4.09–2.62 V, moderately high field-effect mobility rates of 3.36 × 10−2∼2.32 × 10−1 cm2 V−1 s−1, and high ON/OFF ratios of approximately 105. This study opens a route towards transparent and highly stable hybrid dielectric materials with tunable dielectric properties, by careful selection of NPs and polymer matrix combinations.

 Please wait while we load your content...
Please wait while we load your content...