Investigations on structural and optical properties of ZnO and ZnO:Co nanoparticles under dense electronic excitations†
Abstract
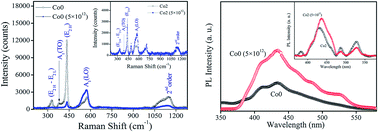
In the present study, the structural, morphological, and optical properties of Co-doped ZnO nanoparticles (NPs) prepared by a sol–gel method before and after dense electronic excitations caused by swift heavy ion irradiation have been reported. The pristine and ZnO:Co NPs were irradiated by using a 200 MeV Ag15+ ion beam at a fluence of 5 × 1012 ions per cm2. Structural characterization has been performed using X-ray diffraction (XRD) with Rietveld refinement. It shows that the samples are of single phase; grain size and tensile strain has been increased in the ion-irradiated samples. Room temperature Raman spectroscopy measurements show that microscopic structural disorders reduce the translational symmetry giving rise to local distortions in the lattice. Atomic force microscopic (AFM) studies show prominent grain boundaries and suggest that roughness of the irradiated surfaces increases strongly compared to their pristine counterparts. Optical absorption and photoluminescence (PL) studies also reflect the dopant incorporation and swift heavy ion (SHI) irradiation effect on the nanoparticles. UV-Vis absorption measurement has been utilized to estimate the optical bandgap of pristine and irradiated ZnO and Co-doped ZnO nanoparticles. Enhancement in the PL intensity has been observed in the irradiated samples with respect to their pristine counterparts which can be explained on the basis of the increase of different defect states and Zn–O bonds on the surfaces of the irradiated nanoparticles arising from surface modification. Grain boundaries have played an important role in the optical properties (absorption and PL).

 Please wait while we load your content...
Please wait while we load your content...