Morphology and wettability control of honeycomb porous films of amphiphilic fluorinated pentablock copolymers via breath figure method†
Zhiguang Liab,
Xiaoyan Ma*ab,
Duyang Zang*a,
Beirong Shangab,
Xiu Qiangab,
Qing Hongab and
Xinghua Guanab
aKey Laboratory of Space Applied Physics and Chemistry, Ministry of Education, School of Science, Northwestern Polytechnical University, Xi'an 710129, Shaanxi Province, China. E-mail: m_xiao_yana@nwpu.edu.cn; Tel: +86-29-88431676
bKey Laboratory of Polymer Science and Technology, School of Science, Northwestern Polytechnical University, Xi'an 710129, Shaanxi Province, China. E-mail: dyzang@nwpu.edu.cn; Tel: +86-29-88431618
First published on 19th September 2014
Abstract
Amphiphilic pentablock copolymers of poly (trifluoroethyl methacrylate)-b-poly (methyl methacrylate)-b-poly (ethylene glycol)-b-poly (methyl methacrylate)-b-poly (trifluoroethyl methacrylate) (PTFEMA-b-PMMA-b-PEG-b-PMMA-b-PTFEMA) with three different block ratios as well as molecular weights were used to fabricate honeycomb structured porous films through the breath figure technique. Several critical influencing factors such as macromolecular structures, solvent properties, copolymer concentrations and the substrates were investigated to control the morphology of the pores. The results showed that chloroform utilized as a solvent with an appropriate concentration of 45 mg mL−1 on the substrate of silicon wafer offered the optimum condition. It could be evidenced that the pore sizes of the honeycomb films were increased by enhancing the molecular weight of the copolymer. In addition, an increase in the copolymer concentration leads to a decrease in the pore size, and even causes a few porous structures to disappear. The viscosity of the liquid substrates also affects the pore size, and it was found that a larger pore size was formed with an increase in the viscosity. The porous films possessed better hydrophobic and oleophobic properties than the flat films. In addition, the pincushion structure films exhibited the best hydrophobic and oleophobic properties and an excellent water-adhesion ability in the reverse state. This work may facilitate our understanding of the breath figure process and assist us in preparing films under different conditions, which show different perspectives as micro-suckers, hydrophobic and oleophobic membranes.
1. Introduction
Microporous films with a regular and ordered patterned structure demonstrate an attractive topic of research, especially for their potential applications such as in superhydrophobic polymer films, filter membranes, catalysts, cell culture substrates and optical materials.1–7 The breath figure (BF) method has been regarded as a powerful tool for the fabrication of strictly ordered and closely packed 2D and 3D nano- and microstructures8,9 due to its facile, economical, nontemplated and versatile nature10,11 to prepare films with ordered honeycomb structures, in which the template consists of an ordered array of water droplets that may be removed by simple evaporation. Generally, the BF process involves three steps: the evaporation of a volatile solvent from a polymer solution in the presence of a humid atmosphere, the accumulation of water vapor molecules as well as the condensation of water droplets, and finally the sacrifice of the water droplets template to form the porous films.The driving forces of the accumulation of water vapor molecules and the condensation of water droplets are considered to be the capillary force and the surface convection resulting from the temperature gradient generated between the humid atmosphere and the casting solution, which is caused by the solvent evaporation of the casting solution. The final water droplets with a tunable diameter in the range from 0.2 to 10 μm12 serve as sacrificial templates prior to the complete solidification of the polymer solution, which leads to a highly ordered hexagonal array honeycomb patterned film after the evaporation of both the solvent and water due to Marangoni convection.12,13 The key points in this procedure are to prevent the coalescence of water droplets and precipitation of the polymer on the solution surface, which could be affected by the nature of the solvent, macromolecular structures, relative humidity of ambience, the polymer concentrations and substrates.1,14–20
The properties of solvent, such as insolubility in water and volatility determine whether the BF films can be formed perfectly. The most common solvents used for the BF technique include carbon disulfide and chloroform due to their proper volatility as well as insolubility.13,21 On the other hand, the water-miscible solvents such as THF are not suitable for the fabrication of ordered porous films by the BF method because of their affinity with water. However, Li et al.22 and Hu's group14 utilized poly(L-lactide) and PS-b-PAA as copolymer matrix, respectively, and THF as the solvent can also fabricate honeycomb porous films. Therefore, the effect of solvent on the formation of honeycomb porous films is also related to the chemical structures. As a result, the macromolecular structure is a crucial element to determine the final morphology of the films. During the past two decades, it has been shown that various types of polymers with different macromolecular structures, such as rod–coil copolymers, star shaped polymers, comb copolymers, graft polymers, supramolecular polymers and dendritic polymers can be fabricated into honeycomb patterned films with a controlled pore size.17,23–29 The polar groups, degree of branching and end-groups of the polymers can affect the precipitation and stabilization of the water droplets, and then influence the pore size. Furthermore, relative humidity of ambience is another factor which influences the water condensation on the solution surface, and consequently determines the pore size and regularity of the pore arrangement. Environments with a relative humidity of 50% or higher are necessary to promote favorable condensation.15,30 In particular, a trend is observed in which the size of the pores in the films increases almost in a linear fashion with a higher humidity (>60%) as Hu et al. proposed.14 Moreover, the pore size is also considerably influenced by the polymer concentrations, which stabilize water droplets and determine the total contact area between the water phase and polymer solution phase.31 In addition, Stenzel32 observed a strong relationship (R = K/c, K is a constant dependent on the polymer material used) between the pore size (R) and the concentration (c) in solution while using amphiphiles, whereas less influence was observed while using various concentrations of star polymers.33 Besides, various studies have shown that the substrate has an effect on the regularity and final quality of the pore array in the films. Billon and co-workers34 fabricated a more regular organization in mica using poly(butyl acrylate)-b-polystyrene (PBA-b-PS) as a polymer, which is due to the electrostatic interactions between the cationic ionomer ends and the oxyanions of the mica surface. Xi et al.31 also found that mica was the best substrate for the casting of dendronized block copolymers in the BF method, but honeycomb films could not be successfully prepared on silicon wafer. However, honeycomb porous thin films of PS-b-PAA were obtained on silicon wafer by Hu's group.14 These phenomena can be ascribed to the different affinity of water and polymer solutions with the substrates.
Based on the above mentioned descriptions, we can observe that various explanations for the controlled arrangement of ordered patterns by the BF technology have been proposed.35–39 However, the possible interplay of general mechanisms for the formation of all BF systems is complex, since each casting system is unique, and in some cases, no definite conclusions on the effect of each variable can be drawn. The complexity of the process with its manifold influences does not readily allow an absolute prediction, and even the developed empirical relationships merely work well in certain systems.40 Furthermore, the identification of the crucial factors directing the formation is still a matter of debate, and subtle changes in the casting conditions can change the outcome of the film preparation significantly because of the complicated character of BF formation.41
The above controversial results confirm that it is of great significance to explore the key factors for the formation of BF through a variety of polymers as film-forming materials. In the present work, amphiphilic pentablock copolymers with PMMA and PTFEMA as hydrophobic segments and PEG as hydrophilic segment were utilized to prepare the porous films and to explore the mechanism of the BF process. As versatile building blocks, PTFEMA is a kind of special polymer with unique properties including thermal and chemical resistance and a lower surface energy, and thus have been extensively applied in many fields.42,43 However, the low solubility, difficult processability and the high costs will certainly limit its applications. Thus, the introduction of PMMA and PEG to the PTFEMA can help reduce costs and improve the solubility and processability. In addition, PEG has attracted considerable attention because of its hydrophilicity and functional flexibility. Based on the pentablock copolymers, we expected to obtain the honeycomb porous films through the BF method with various excellent properties such as thermal and chemical resistance, hydrophobicity and oleophobicity.
Until now, more fluorinated copolymers are chosen for hydrophobic applications, but the hydrophobic and oleophobic performances still have much scope for improvement. The introduction of surface morphologies of honeycomb porous films, which enhanced the surface roughness, will be concerned to solve this problem. Chen et al.27 fabricated highly ordered porous films from crystalline conjugated rod–coil diblock copolymers of PF-b-PSA (poly[2,7-(9,9-dihexylfluorene)]-b-poly(stearyl acrylate)) through the BF process, and the water contact angle was over 160° when the top layer was peeled off. Similarly, Yabu et al.6 prepared a superhydrophobic and oleophobic surface from fluorinated polymers by the BF method. It was found that the contact angles of porous and pin-cushion films were 145° and 170°, respectively, which are much higher than the contact angle (117°) of the relative flat film. This surface can be widely applied in self-cleaning surfaces, biological fields, and so forth. To further expand the application of the honeycomb porous films of the pentablock copolymers, the wettability, hydrophobic and oleophobic properties are investigated in this paper.
2. Experimental
2.1. Materials
The pentablock copolymers of PTFEMA-b-PMMA-b-PEG-b-PMMA-b-PTFEMA with three different molecular weights were synthesized through a one pot method via atom transfer radical polymerization. The synthesis processes is shown in Fig. S1 and S2 in ESI.† The pentablock copolymers were analyzed via FTIR and NMR, as shown in Fig. S3 and S4 in ESI.† The GPC analysis results are listed in Table 1. Water used in all of the experiments was de-ionized and ultrafiltrated to 18.2 MΩ with an ELGA Lab water system. All other reagents (benzene (C6H6), chloroform (CHCl3), dichloromethane (CH2Cl2), tetrahydrofuran (THF) and n-dodecane) were analytically pure and used without further purification.| Serial number | Block ratio PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMMA PMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTFEMA PTFEMA |
Mn (g mol−1) | PDI |
|---|---|---|---|
| Copolymer-1 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
1.49 |
| Copolymer-2 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
1.58 |
| Copolymer-3 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.57 |
2.2. Preparation of the copolymer films
To prepare honeycomb porous films, the pentablock copolymers were dissolved in CHCl3, CH2Cl2, C6H6 and THF, and then cast onto silicon wafer, ITO glass or stainless steel under a flow of moist air. The airflow was vertically blown over the solution surface. The solvent started to evaporate and water vapor condensed onto the solution surface simultaneously. After completely evaporating the solvent and water, the porous films were prepared. For comparison, nonporous films were also cast from CHCl3 solution of the pentablock copolymer and dried under normal conditions.2.3. Characterization
Scanning Electron Microscopy (SEM) images were used to observe apparent morphologies of porous films, which were carried out on VEGA 3 LMH (Česko TESCAN) with an accelerating voltage of 10 kV. Contact angles were measured using ultra-pure water or copolymer solution by the pendent drop method with a JC2000D4 Powereach Tensionmeter made by Shanghai Zhongchen Company. The copolymer solutions (45 mg mL−1 in CHCl3) were casted on glass slides (43 × 17 × 16 mm3), and the wetting liquid used was water. A microsyringe was used to deliver water to the film surface. For each angle reported, at least ten sample readings from different surface locations were averaged.3. Results and discussion
3.1. Factors influencing the honeycomb porous films
It is noteworthy that the morphologies, pore size and regularity of the pores prepared from the BF method can be tailored by changing some conditions that affect the fabrication of the honeycomb porous films.17–19,44,45 In this section, some critical factors such as macromolecular structures, solvent properties, copolymer concentrations and substrates on the morphology of porous films are investigated.The SEM images of porous films of PEG macroinitiator, copolymer-1, copolymer-2 and copolymer-3 with different block ratios and molecular weights prepared with the concentration of 45 mg mL−1 in CHCl3 are illustrated in Fig. 1. It is noticeably found that the SEM image of PEG macroinitiator film is a rod-like structure instead of a porous formation in Fig. 1a due to its hydrophilicity and low molecular weight.27 On the contrary, the PMMA and PTFEMA blocks on copolymer with a long alkyl side chain could facilitate the stabilization of the water droplets and result in an ordered porous structure as evidenced in Fig. 1b–d.27 Moreover, the increase in molecular weight of the pentablock copolymer seems to result in the formation of larger pores. The reason is that the higher molecular weight indicates a lower mole fraction and a faster solvent evaporation, which is beneficial for the growth of the condensed water droplets.46 The lower mole fraction copolymer solution may lead to a delayed precipitation and provide enough time for the condensed water droplets to grow and finally form a larger pore size after sacrificing the water droplets.17,18,22
 | ||
| Fig. 2 SEM images of copolymer-1 films dissolved in different solvents with the concentration of polymer at 45 mg mL−1, (a)–(d) are CH2Cl2, CHCl3, THF and C6H6, respectively. | ||
The major differences between these solvents are their evaporation rate and the interactions between the solvent and the water droplets. The fast evaporation rate of the solvent corresponds to the rapid cooling rate of the copolymer solution surface and favors condensation of water vapor. For CH2Cl2 and CHCl3, the solvent of CH2Cl2 may completely disappear from the system before water droplets form a regular packing. On the other hand, the lower volatility of CHCl3 delays the evaporation of the solvent completely. Consequently, the water droplets have more time to grow and sink, resulting in the larger and regular pores in the films17 (Fig. 2a and b).
In addition, water and THF are miscible with each other. When water droplets condense onto the copolymer/THF solution, the copolymer cannot effectively stabilize the droplets, and the droplets diffuse in the solution because of its affinity with THF. However, the viscosity of the solution increases with the evaporation of THF; some droplets are formed without coalescence. Finally, the disordered morphology is formed (Fig. 2c). The C6H6 with low volatility evaporates too slowly to produce enough temperature gradient to allow sufficient surface cooling to condense water on the surface of the solution. As a result, the water droplets can't easily sink into the solution. Consequently, it does not allow the formation of obvious porous structures as illustrated in Fig. 2d. The above results indicate that the solvent is one of the key factors for the ordered arrangement in BF method, which would determine whether the ordered porous films can be formed.
 | ||
| Fig. 3 SEM images of copolymer-1 films, the concentrations of (a)–(f) are 5, 15, 30, 45, 60, 75 mg mL−1. | ||
It is noteworthy that the average pore sizes of the obtained films decrease from 1.59 ± 0.15 μm (CV = 9.4%), 1.39 ± 0.10 μm (CV = 7.2%), 0.94 ± 0.03 μm (CV = 3.2%) to 0.80 ± 0.09 μm (CV = 11.3%) when the copolymer concentrations increase from 15 to 60 mg mL−1, as illustrated in Fig. 4. The correlation between the pore size and the solution concentration is almost a linear variation. The CV is an indication of the variation as well as the degree of order in the pore size. The relatively small coefficient of variation suggests the regularity and the similar distance of any two neighbouring pores. Moreover, the most ordered porous film is obtained in a solution with a concentration of 45 mg mL−1, and the pores are in a regular hexagonal arrangement to reduce free energy.40
 | ||
| Fig. 4 Average pore size as a function of concentration of the porous films prepared from copolymer-1/CHCl3 solutions. Coefficient of variation (CV)27 is a normalized dispersion of a probability distribution. | ||
It is known that the pore size is determined by the size of the condensed water droplet. The pore sizes of water droplets relate to the growth rate and growth time. With the increase in the concentration, the process of phase-inversion from liquid to solid is accelerated, which then shortens the growth time of the droplets.21 On the other hand, the growth rate of the droplets is proportional to the surface temperature gradient (ΔT) between the temperature of solution surface and atmosphere, which is described as49
| dR/dt ∼ ΔT0.8 | (1) |
It is addressed by Henry's Law that the more concentrated solution corresponds to the lower vapor pressure and the slower evaporation rate of the solvent,21,46 which leads to the smaller decrease in ΔT. Therefore, the growing rate and growing time of the water droplets on the solution surface with a higher copolymer concentration are both slower, and the obtained water droplets have a smaller size as a result, which leads to a smaller pore size. In addition, the precipitation rate is the key factor in the formation of the ordered porous film. Generally, a higher copolymer concentration leads to a faster precipitation rate by which the water droplets could be encapsulated and solidified immediately, and the smaller pore size could be formed. Moreover, a higher concentration corresponds to a higher viscosity, which would lead to an increased efficiency of the encapsulation of condensed water droplets. Consequently, the droplets do not have enough time to grow larger,15,22,46 and small pores are formed. The solution with an excessively low concentration (5 mg mL−1) with a viscosity too low to encapsulate the droplets or prevent their coalescence27 resulted in the formation of a disordered and irregular arrangement (Fig. 3a). With the increase in copolymer concentration, the water droplets can be more easily stabilized by the solution, which is in favor of arraying the droplets in an orderly manner. The precipitation rate would be enhanced with the increase in the copolymer concentration,50 which can stabilize water droplets and inhibit them from aggregating to form an ordered porous structure (Fig. 3b–e). In addition, no apparent holes resulted from the high copolymer concentration (75 mg mL−1),19,51 as exhibited by the red arrows in Fig. 3f. The reason is that the highest viscosity of the copolymer solution makes it more difficult for the water droplets to sink into the copolymer solution, consequently resulting in the slower growth of droplets and smaller pores. These results suggest that a suitable copolymer concentration is needed for the formation of the ordered honeycomb structures.
 | ||
| Fig. 5 SEM images of copolymer-1 films dissolved in CHCl3 on different solid substrates: (a) ITO glass, (b) stainless steel, (c) silicon wafer. | ||
It is shown that the pore structures exhibit regular hexagonal arrays on the substrate of silicon wafer with uniform pore sizes of 0.94 ± 0.03 μm (CV = 3.2%) (Fig. 5c), while the pore patterns formed on the ITO glass and the stainless steel are less ordered, with non-uniform pore sizes of 1.72 ± 0.31 μm (CV = 18.0%) and 1.62 ± 0.17 μm (CV = 10.5%) (Fig. 5a and b). The smallest CV value reveals that the substrate of silicon wafer results in an ordered microporous structure. This could be ascribed to the different affinity of water and copolymer solutions with different substrates, which are demonstrated by their contact angles listed in Table 2.
| Solid substrates | ITO glass | Stainless steel | Silicon wafer |
|---|---|---|---|
| CA of water/° | 74.3 ± 2.4 | 67.2 ± 2.1 | 40.5 ± 2.2 |
| CA of copolymer solution/° | 14.2 ± 0.4 | 12.9 ± 0.3 | 9.5 ± 0.3 |
It is evident that it is easy for the water droplets to be adsorbed onto the silicon wafer as a steady template for the formation of porous films. Moreover, it is also facile for the copolymer solution to be spread onto the silicon wafer. The water droplets are easily adsorbed onto the hydrophilic substrates, which causes the template's stability to increase, such that the formation of highly ordered porous structure is feasible. In addition, it is well known that the wetting of solid substrates with copolymer solution is beneficial to the periodicity and regularity of pores.14,31 These processes lead to ordered porous films fabricated on the silicon wafer. On the other hand, the ITO glass with the highest contact angle of the copolymer solution may restrain the spreading behavior of copolymer solutions on the substrates by slowing the nucleation of water droplets at the water/CHCl3 interface, which accordingly yields the largest pore sizes. As a result, average pore size decreases as hydrophilicity of the copolymer solution increases on the substrate.
Compared with the solid surface, the liquid substrate with a large surface tension (e.g., water) would be beneficial to the spreading of the copolymer solution by a simple qualitative analysis. Wan et al.1 prepared honeycomb films on the surface of various organic solvents, and found that the surface tension of the liquids was crucial to the formation of through-pores. However, there is no research on the viscosity of liquid substrates, which influences the spread and fluidity. In this work, dimethyl silicone with four different viscosities are selected as substrates and investigated to analyze the morphology, and the results are depicted in Fig. 6.
 | ||
Fig. 6 SEM images of the pentablock copolymer film prepared at the dimethyl silicone surfaces. The viscosity is (a) 10, (b) 100, (c) 1000 and (d) 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. | ||
It is noteworthy that with the increase in the viscosity of dimethyl silicone, the average pore size increased. Specifically, a hexagonal pore morphology is observed (Fig. 6d) in the film prepared from the dimethyl silicone with a viscosity of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, which is different from the circular pores in the films prepared from the dimethyl silicone with viscosities of 10, 100 and 1000. The copolymer solution spread well at the interface of dimethyl silicone with a lower viscosity because of its better fluidity. The water droplets are beneficial to precipitate at the copolymer surface due to the short evaporation time of the solvent, consequently resulting in the slower growth of water droplets and the formation of smaller pores and vice versa.
000, which is different from the circular pores in the films prepared from the dimethyl silicone with viscosities of 10, 100 and 1000. The copolymer solution spread well at the interface of dimethyl silicone with a lower viscosity because of its better fluidity. The water droplets are beneficial to precipitate at the copolymer surface due to the short evaporation time of the solvent, consequently resulting in the slower growth of water droplets and the formation of smaller pores and vice versa.
Based on the analysis mentioned above, one can draw a conclusion that the formation of the honeycomb porous film of the pentablock copolymer would be affected by the molecular weight, solvent and copolymer concentration, and the regularity of the pores is influenced by the use of an appropriate solvent and substrate. In addition, the molecular weight and copolymer concentration have an effect on the pore size.
3.2. Wettability of the copolymer films
Copolymers composed of fluorinated acrylate have been broadly applied and proven to possess excellent surface performance, hydrophobic and oleophobic properties,43 but how the wettability can be affected, and whether superhydrophobic films could be obtained are our concerns. To solve these problems, water and n-dodecane contact angles of flat films and related porous and peeled films are summarized in Fig. 7.It is clearly shown that the contact angles of the three copolymers increased to 95.98°, 89.13° and 79.17° when flat films are deposited (Fig. 7), which is higher than that of the glass (45.64°) due to the intrinsic hydrophobic properties of the copolymer. It is well known that the low surface energy of the surface could result in hydrophobic effects.
Surface energy can be calculated from contact angle measurements by the Owens and Wendt method. The equilibrium contact angle is well defined by Young's equation,54
σs = σsl + σl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (2) |
| σl = σdl + σpl | (3) |
| σs = σds + σps | (4) |
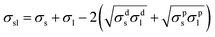 | (5) |
substituting the σsl in eqn (3) with (6), we get
 | (6) |
If the contact angles of two different liquids on the same polymer surface are known, and σds and σps can be obtained from eqn (6), the surface energy of the polymer film can be calculated using eqn (4). In this paper, ultra-pure water and n-dodecane are selected as the probe liquids to determine the surface energies of copolymer films. The values of σdl (23.9 mN m−1) and σpl (48.8 mN m−1) for water and σdl (23.9 mN m−1) and σpl (0 mN m−1) for n-dodecane are used in the calculation. The resultant surface energies (σS) of the flat films of the three copolymers are summarized in Table 3.
| Solid substrates | σds (mN m−1) | σps (mN m−1) | σs (mN m−1) |
|---|---|---|---|
| Copolymer-1 | 17.47 | 3.01 | 20.48 |
| Copolymer-2 | 19.96 | 4.64 | 24.60 |
| Copolymer-3 | 22.25 | 8.29 | 30.54 |
It is obvious that the highest fluorinated segment of copolymer-1 results in the lowest surface energy. As a result, one can observe that the surface energy decreases with the increasing amount of fluorinated segments, which results in stronger hydrophobic properties.
Compared with the flat films, the contact angles of the honeycomb films from copolymer-1 to copolymer-3 are improved to 117.6°, 109.23° and 105.33° due to the presence of the highly ordered honeycomb pattern, which enhances the surface roughness and amplifies the hydrophobic properties.23,34 In addition, no matter for flat films or porous films, copolymer-1 always possesses higher values of water contact angles than that of copolymer-2 and copolymer-3, due to the increased hydrophobicity and fluorinated segments.18
To further promote the roughness effect, the top layer of the honeycomb porous film is peeled off using an adhesive tape to obtain a pincushion structure (Fig. 8a). It is clearly shown that the pincushion structure from the three copolymers can enhance the hydrophobicity and raise the contact angles of water to 140.63°, 135.20° and 131.12°, respectively. The fluorine content of the copolymers is slightly low, which makes them unable to impart excellent water repellence. Consequently, the contact angles cannot reach superhydrophobic values. Compared with the honeycomb film, the increased contact angle is due to the increased surface roughness of the pincushion structure. Based on the analysis, we can derive that surface hydrophobicity is mainly caused by the chemical heterogeneity and geometrical roughness of the surface.55,56 The relationship between the rough topography and the contact angle is illustrated by the Cassie–Baxter equation:57–60
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θr = f1 θr = f1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ − f2 θ − f2
| (7) |
 | ||
| Fig. 9 Fractions of the solids on the porous and peeled films of (a) copolymer-1, (b) copolymer-2 and (c) copolymer-3. | ||
It is noteworthy that the pincushion structure also exhibited excellent water-adhesion ability. The water droplet inhibits rolling and sticks firmly on the pincushion structure film when the film is reversed, as presented in Fig. 8b, which is affected by the van der Waals force or hydrogen bonding between the water molecules and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the copolymer.61–63 In fact, air in the pores is trapped by the copolymer walls and the bottom of the water droplet (green parts in Fig. 8c). This closed air volume in the pincushion configuration induces a capillary-like force to maintain the water in suspension, which acts as micro-suckers.34 It is important to note that the chemical composition and pincushion structure play a critical role in exhibiting hydrophobicity and strong adhesion with water.
O groups of the copolymer.61–63 In fact, air in the pores is trapped by the copolymer walls and the bottom of the water droplet (green parts in Fig. 8c). This closed air volume in the pincushion configuration induces a capillary-like force to maintain the water in suspension, which acts as micro-suckers.34 It is important to note that the chemical composition and pincushion structure play a critical role in exhibiting hydrophobicity and strong adhesion with water.
The honeycomb porous films possess an oleophobic property as shown in Fig. 7. The trend of the oleophobic property is similar to the hydrophobic property, which is related to the fluorinated segment and the surface structure. Similarly, the oleophobicity is affected by both the surface chemistry and the surface roughness, which originate from intrinsic oleophobic properties of the copolymer and microstructures of the films.
4. Conclusions
Highly ordered honeycomb films have been successfully prepared from the amphiphilic pentablock copolymers composed of PEG, PMMA and PTFEMA by the breath figure method. The pore sizes and regularity of the honeycomb porous films are influenced by macromolecular structures, solvent properties, copolymer concentrations and substrates. It is demonstrated that CHCl3 utilized as a solvent at a suitable concentration of 45 mg mL−1 on the substrate of silicon wafer offers the optimum condition. Moreover, the pore sizes of honeycomb films are decreased with the increasing concentration of the copolymer solution and the decreasing molecular weight. The viscosity of the liquid substrates also affects the pore size, and it was found that a larger pore size is formed with an increase in the viscosity. Furthermore, the increase in the amount of fluorinated segments and surface roughness results in stronger hydrophobic and oleophobic properties. In addition, the pincushion structure films have excellent water-adhesion ability.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 51301139), Natural Science Foundation of Shaanxi Province (Grant no. 2012JQ1016), Innovation Project of Science and Technology of Shaanxi Province (Grant no. 2013KTCG01-14) and NPU Foundation for Fundamental Research (NPU-FFR-JCY20130147).References
- L.-S. Wan, J.-W. Li, B.-B. Ke and Z.-K. Xu, J. Am. Chem. Soc., 2012, 134, 95–98 CrossRef CAS PubMed.
- X. Wu and S. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4966–4975 CAS.
- C. Du, A. Zhang, H. Bai and L. Li, ACS Macro Lett., 2012, 2, 27–30 CrossRef.
- D. Beattie, K. H. Wong, C. Williams, L. A. Poole-Warren, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Biomacromolecules, 2006, 7, 1072–1082 CrossRef CAS PubMed.
- H. Cong, J. Wang, B. Yu and J. Tang, Soft Matter, 2012, 8, 8835–8839 RSC.
- H. Yabu, M. Takebayashi, M. Tanaka and M. Shimomura, Langmuir, 2005, 21, 3235–3237 CrossRef CAS PubMed.
- E. Bormashenko, S. Balter, Y. Bormashenko and D. Aurbach, Colloids Surf., A, 2012, 415, 394–398 CrossRef CAS PubMed.
- L.-S. Wan, L.-W. Zhu, Y. Ou and Z.-K. Xu, Chem. Commun., 2014, 50, 4024–4039 RSC.
- H. Bai, C. Du, A. Zhang and L. Li, Angew. Chem., Int. Ed., 2013, 52, 12240–12255 CrossRef CAS PubMed.
- A. Gugliuzza and E. Drioli, J. Membr. Sci., 2007, 300, 51–62 CrossRef CAS PubMed.
- A. Gugliuzza, M. C. Aceto, F. Macedonio and E. Drioli, J. Phys. Chem. B, 2008, 112, 10483–10496 CrossRef CAS PubMed.
- M. Srinivasarao, D. Collings, A. Philips and S. Patel, Science, 2001, 292, 79–83 CrossRef CAS PubMed.
- J. Peng, Y. Han, Y. Yang and B. Li, Polymer, 2004, 45, 447–452 CrossRef CAS PubMed.
- C. Wang, Y. Mao, D. Wang, Q. Qu, G. Yang and X. Hu, J. Mater. Chem., 2008, 18, 683–690 RSC.
- W. Liu, R. Liu, Y. Li, W. Wang, L. Ma, M. Wu and Y. Huang, Polymer, 2009, 50, 2716–2726 CrossRef CAS PubMed.
- A. Honglawan and S. Yang, Soft Matter, 2012, 8, 11897–11904 RSC.
- X.-Y. Li, Q.-L. Zhao, T.-T. Xu, J. Huang, L.-H. Wei and Z. Ma, Eur. Polym. J., 2014, 50, 135–141 CrossRef CAS PubMed.
- Y. Xue, H.-C. Lu, Q.-L. Zhao, J. Huang, S.-G. Xu, S.-K. Cao and Z. Ma, Polym. Chem., 2013, 4, 307–312 RSC.
- C.-X. Liu, W.-Z. Lang, B.-B. Shi and Y.-J. Guo, Mater. Lett., 2013, 107, 53–55 CrossRef CAS PubMed.
- U. H. F. Bunz, Adv. Mater., 2006, 18, 973–989 CrossRef CAS.
- B. Zhao, J. Zhang, H. Wu, X. Wang and C. Li, Thin Solid Films, 2007, 515, 3629–3634 CrossRef CAS PubMed.
- B. Zhao, J. Zhang, X. Wang and C. Li, J. Mater. Chem., 2006, 16, 509–513 RSC.
- J. Chen, X. Yan, Q. Zhao, L. Li and F. Huang, Polym. Chem., 2012, 3, 458–462 RSC.
- L.-W. Zhu, Y. Ou, L.-S. Wan and Z.-K. Xu, J. Phys. Chem. B, 2014, 118, 845–854 CrossRef CAS PubMed.
- Y.-A. Su, W.-F. Chen, T.-Y. Juang, W.-H. Ting, T.-Y. Liu, C.-F. Hsieh, S. A. Dai and R.-J. Jeng, Polymer, 2014, 55, 1481–1490 CrossRef CAS PubMed.
- L.-W. Zhu, L.-S. Wan, J. Jin and Z.-K. Xu, J. Phys. Chem. C, 2013, 117, 6185–6194 CAS.
- Y.-C. Chiu, C.-C. Kuo, C.-J. Lin and W.-C. Chen, Soft Matter, 2011, 7, 9350–9358 RSC.
- L. A. Connal, R. Vestberg, P. A. Gurr, C. J. Hawker and G. G. Qiao, Langmuir, 2008, 24, 556–562 CrossRef CAS PubMed.
- K. H. Wong, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Polymer, 2007, 48, 4950–4965 CrossRef CAS PubMed.
- X. Li, Y. Wang, L. Zhang, S. Tan, X. Yu, N. Zhao, G. Chen and J. Xu, J. Colloid Interface Sci., 2010, 350, 253–259 CrossRef CAS PubMed.
- C. X. Cheng, Y. Tian, Y. Q. Shi, R. P. Tang and F. Xi, Langmuir, 2005, 21, 6576–6581 CrossRef CAS PubMed.
- M. H. Stenzel, Aust. J. Chem., 2002, 55, 239–243 CrossRef CAS.
- X. Qiang, X. Ma, Z. Li and X. Hou, Colloid Polym. Sci., 2014, 292, 1531–1544 CAS.
- L. Ghannam, M. Manguian, J. François and L. Billon, Soft Matter, 2007, 3, 1492–1499 RSC.
- H. Yamazaki, K. Ito, H. Yabu and M. Shimomura, Soft Matter, 2014, 10, 2741–2747 RSC.
- R. Daly, J. E. Sader and J. J. Boland, Soft Matter, 2013, 9, 7960–7965 RSC.
- J. Blaschke, T. Lapp, B. Hof and J. Vollmer, Phys. Rev. Lett., 2012, 109, 068701 CrossRef.
- E. Servoli, G. A. Ruffo and C. Migliaresi, Polymer, 2010, 51, 2337–2344 CrossRef CAS PubMed.
- E. Bormashenko, A. Musin, Y. Bormashenko, G. Whyman, R. Pogreb and O. Gendelman, Macromol. Chem. Phys., 2007, 208, 702–709 CrossRef CAS.
- M. H. Stenzel, C. Barner-Kowollik and T. P. Davis, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2363–2375 CrossRef CAS.
- J. Ding, A. Zhang, H. Bai, L. Li, J. Li and Z. Ma, Soft Matter, 2013, 9, 506–514 RSC.
- A. Bruno, Macromolecules, 2010, 43, 10163–10184 CrossRef.
- N. M. L. Hansen, M. Gerstenberg, D. M. Haddleton and S. Hvilsted, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 8097–8111 CrossRef CAS.
- E. Ferrari, P. Fabbri and F. Pilati, Langmuir, 2011, 27, 1874–1881 CrossRef CAS PubMed.
- W. Dong, Y. Zhou, D. Yan, Y. Mai, L. He and C. Jin, Langmuir, 2009, 25, 173–178 CrossRef CAS PubMed.
- Y. Xu, B. Zhu and Y. Xu, Polymer, 2005, 46, 713–717 CrossRef CAS PubMed.
- Y. Tian, Q. Jiao, H. Ding, Y. Shi and B. Liu, Polymer, 2006, 47, 3866–3873 CrossRef CAS PubMed.
- Y. Tian, H. Ding, Q. Jiao and Y. Shi, Macromol. Chem. Phys., 2006, 207, 545–553 CrossRef CAS.
- D. Beysens, A. Steyer, P. Guenoun, D. Fritter and C. Knobler, Phase Transitions, 1991, 31, 219–246 CrossRef CAS.
- L. A. Connal, P. A. Gurr, G. G. Qiao and D. H. Solomon, J. Mater. Chem., 2005, 15, 1286–1292 CAS.
- M. Huh, M.-H. Jung, Y. S. Park, T.-B. Kang, C. Nah, R. A. Russell, P. J. Holden and S. I. Yun, Polym. Eng. Sci., 2012, 52, 920–926 CAS.
- J. Wang, H.-X. Shen, C.-F. Wang and S. Chen, J. Mater. Chem., 2012, 22, 4089–4096 RSC.
- M. H. Nurmawati, R. Renu, P. K. Ajikumar, S. Sindhu, F. C. Cheong, C. H. Sow and S. Valiyaveettil, Adv. Funct. Mater., 2006, 16, 2340–2345 CrossRef CAS.
- K.-J. Lian, C.-Q. Chen, H. Liu, N.-X. Wang, H.-J. Yu and Z.-H. Luo, J. Appl. Polym. Sci., 2011, 120, 156–164 CrossRef CAS.
- T.-Y. Han, J.-F. Shr, C.-F. Wu and C.-T. Hsieh, Thin Solid Films, 2007, 515, 4666–4669 CrossRef CAS PubMed.
- D. Zang, F. Li, X. Geng, K. Lin and P. Clegg, Eur. Phys. J. E: Soft Matter Biol. Phys., 2013, 36, 1–8 CrossRef PubMed.
- S. Yang, S. Chen, Y. Tian, C. Feng and L. Chen, Chem. Mater., 2008, 20, 1233–1235 CrossRef CAS.
- E. Bormashenko, S. Balter and D. Aurbach, Macromol. Chem. Phys., 2012, 213, 1742–1747 CrossRef CAS.
- B.-B. Ke, L.-S. Wan, P.-C. Chen, L.-Y. Zhang and Z.-K. Xu, Langmuir, 2010, 26, 15982–15988 CrossRef CAS PubMed.
- B.-B. Ke, L.-S. Wan, Y. Li, M.-Y. Xu and Z.-K. Xu, Phys. Chem. Chem. Phys., 2011, 13, 4881–4887 RSC.
- N. Zhao, Q. Xie, X. Kuang, S. Wang, Y. Li, X. Lu, S. Tan, J. Shen, X. Zhang, Y. Zhang, J. Xu and C. C. Han, Adv. Funct. Mater., 2007, 17, 2739–2745 CrossRef CAS.
- W.-H. Ting, C.-C. Chen, S. A. Dai, S.-Y. Suen, I. K. Yang, Y.-L. Liu, F. M. C. Chen and R.-J. Jeng, J. Mater. Chem., 2009, 19, 4819–4828 RSC.
- L. Heng, X. Meng, B. Wang and L. Jiang, Langmuir, 2013, 29, 9491–9498 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08472a |
| This journal is © The Royal Society of Chemistry 2014 |



