Pure-silica ZSM-22 zeolite rapidly synthesized by novel ionic liquid-directed dry-gel conversion†
Haimeng Wen,
Yu Zhou,
Jingyan Xie,
Zhouyang Long,
Wei Zhang and
Jun Wang*
State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 210009, China. E-mail: junwang@njtech.edu.cn; Fax: +86-25-83172261; Tel: +86-25-83172264
First published on 22nd September 2014
Abstract
Shortening the crystallization process of zeolites is significant for their applications because of the energy savings. Here, we report a rapid synthetic route for pure-silica ZSM-22 zeolite with TON topology. The synthesis was achieved by dry-gel conversion, where the dry gel was prepared under an unusual acidic condition for hydrolyzing the silica precursor with 1,3-alkylimidazolium ionic liquid as the structure-directing agent (SDA). Highly crystallized pure-silica ZSM-22 can be synthesized within 2 days of crystallization, dramatically shorter than 10% of the conventional hydrothermal strategy. Using a similar procedure, Al-containing ZSM-22 can also be synthesized by simply adding aluminum salt in the initial gel. In addition, understanding of the structure-directing role of ionic liquids is attempted through characterizations of IR, TG, 1H and 13C NMR spectra.
Introduction
Zeolites are a vast class of crystalline porous materials that have been widely used as solid catalysts, ion exchangers, adsorbents and so on.1 Zeolite synthesis usually goes through a hydrothermal treatment process with high temperatures, consuming large amounts of energy. How to shorten the synthetic cycle, especially the high-temperature hydrothermal process, is crucial for practical applications of zeolites, which in turn strongly depend on developing new synthetic strategies. Much effort has been focused on aluminosilicate zeolites,2 but rare attention has thus far been paid to pure silica ones.Pure-silica zeolites are of primary interest in both academic areas and practical applications due to their pure-silica framework, high hydrothermal stability, superior mechanical strength and hydrophobicity.3 In addition, they are accessible in special applications such as separating nonpolar from polar molecules owing to their electroneutral framework.4 To the present, aside from the most widely studied silicalite-1,5 only a few types of zeolitic topologies can be achieved with a pure-silica framework, requiring deboronated borosilicate zeolite as seeding,6 complex templates7 or additives such as fluoride.8 Among various zeolites, ZSM-22 is a TON topology with a one-dimensional pore system (0.45 × 0.55 nm),9 and has exhibited high catalytic activity and selectivity in paraffin isomerization,10 butene isomerization,11 aromatization reaction,12 and so on. Synthesis of the pure phase of ZSM-22 is still difficult, which usually employs a dynamic hydrothermal method and needs vertical speed higher than 400 rpm to avoid the formation of the often-combined impurity phases.13 Meanwhile, Al-containing ZSM-22 can be synthesized in a short time,14 but the synthesis of pure-silica ZSM-22 without impurities usually requires several weeks or even months for complete crystallization.15
Herein, we report a rapid synthesis of pure-silica ZSM-22 via a dry-gel conversion (DGC) method using ionic liquid (1-butyl-3-methylimidazolium bromide, [BMIm]Br) as the structure-directing agent. Ionic liquids (ILs) are applied not only in chemistry but also in materials science due to their versatile properties.16 When applied in zeolite synthesis, ionic liquids have been used as structure-directing agents for the formation of MFI, BEA and ANA topologies,17 and imidazolium-derived ionic liquids can be structurally manipulated to show good structure-directing effects for pentasil zeolites.18 A series of diquaternary organo-cations built on N-methylimidazole has been used for synthesizing TON-type zeolites with SiO2/Al2O3 molar ratios of 50–300,19 and recently 1,3-alkylimidazolium-related ionic liquids were used for synthesizing Al-containing TON topologies, which was not successful for the pure-silica one.20
The DGC method is an efficient route to synthesize zeolites because of its specific advantages. Unlike conventional hydrothermal routes where the materials directly contact water, DGC allows the solid gels to contact only steam and inhibit the potential of phase separation.21 The use of homogeneous gels benefits formation of the pure phase through preventing nonhomogeneous crystallization that often occurs in the conventional static hydrothermal method.22 Further, the high concentration of silica precursor in dry gel facilitates rapid nucleation and thus shortens the crystallization process. As far as we know, the ionic liquid-directed DGC route has not been reported before. In this work, the acid-hydrolysis route is used to prepare the dry gel.23 Correspondingly, pure-silica ZSM-22 is rapidly synthesized with only 2 days for crystallization. The other 1,3-alkylimidazolium-related ionic liquids with 2, 6, 8, 10, 12 and 14 carbons ([EMIm]Br, [HMIm]Br, [OMIm]Br, [DMIm]Br, [C12MIm]Br and [C14MIm]Br) are also investigated. In addition, Al-containing ZSM-22 is also synthesized by adding aluminum salt to the initial gel. Primary understanding of the structure-directing effect was gained through comparative experiments, as well as the IR, TG, 1H and 13C NMR analysis, although the exact crystallization mechanism of the present synthesis is as yet unclear.
Experimental section
Synthesis
All chemicals in this study were analytical grade and used as received except the laboratory-made NaOH solution. A typical procedure for the pure-silica zeolite synthesis was carried out as follows. First, the concentrated sulfuric acid (98 wt%) was slowly dripped into distilled water (1000 mL) to obtain an acidic solution (pH = 1.0). Then the acidic solution (25.20 g) and tetraethylorthosilicate (TEOS, 7.39 g) were mixed in a 100 mL glass beaker under vigorous stirring, and the obtained mixture was stirred at 298 K for 24 h for further hydrolysis of TEOS. To get a basic gel, [BMIm]Br was added into the above mixture, followed by the dropwise addition of NaOH solution (12.5 mol L−1). The compositional molar ratio of the gel is 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x[BMIm]Br
x[BMIm]Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yNa2O
yNa2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40H2O, in which x denotes the molar ratio of IL/SiO2, and y denotes the molar ratio of Na2O/SiO2. Next, aging was conducted in a microwave reactor (CEM, Mars 240/50) at 333 K for 5 h. After aging, the gel was oven-dried at 373 K for 4 h and ground to powdered dry gel. Finally, the dry gel (0.5 g), which is amorphous as seen in the XRD pattern of Fig. S1,† was placed and sealed in a raised Teflon holder inside a 50 mL Teflon-lined stainless steel autoclave involving distilled water (0.5 g) at the bottom for static crystallization at 443 K for 2 days under autogenous pressure. After crystallization the resultant solid was filtered, washed with ethanol and distilled water, and dried at 373 K for 12 h to give the as-synthesized samples. The pure-silica ZSM-22 is obtained by calcining the as-synthesized sample at 823 K for 5 h in air stream to remove the ionic liquid. The obtained pure-silica ZSM-22 samples were named ZX-Y, in which X = 100 × x, and Y = 100 × y. Al-containing ZSM-22 (Al-ZSM-22) was synthesized similarly except that aluminum sulfate was added before [BMIm]Br, and the obtained samples were named ZX-Y-Z, in which the second Z denotes the molar ratio of SiO2/Al2O3 in the gel, and X and Y are defined as before.
40H2O, in which x denotes the molar ratio of IL/SiO2, and y denotes the molar ratio of Na2O/SiO2. Next, aging was conducted in a microwave reactor (CEM, Mars 240/50) at 333 K for 5 h. After aging, the gel was oven-dried at 373 K for 4 h and ground to powdered dry gel. Finally, the dry gel (0.5 g), which is amorphous as seen in the XRD pattern of Fig. S1,† was placed and sealed in a raised Teflon holder inside a 50 mL Teflon-lined stainless steel autoclave involving distilled water (0.5 g) at the bottom for static crystallization at 443 K for 2 days under autogenous pressure. After crystallization the resultant solid was filtered, washed with ethanol and distilled water, and dried at 373 K for 12 h to give the as-synthesized samples. The pure-silica ZSM-22 is obtained by calcining the as-synthesized sample at 823 K for 5 h in air stream to remove the ionic liquid. The obtained pure-silica ZSM-22 samples were named ZX-Y, in which X = 100 × x, and Y = 100 × y. Al-containing ZSM-22 (Al-ZSM-22) was synthesized similarly except that aluminum sulfate was added before [BMIm]Br, and the obtained samples were named ZX-Y-Z, in which the second Z denotes the molar ratio of SiO2/Al2O3 in the gel, and X and Y are defined as before.
For comparison, we also prepared control samples to investigate the structure-directing effect of ionic liquid. We synthesized samples following the above procedure in the absence of [BMIm]Br, which represents the SDA-free route. The structure-directing agent 1,6-hexanediamine (HDA) for the conventional hydrothermal synthesis of ZSM-22 was also used for the present DGC method instead of [BMIm]Br. The gel was basic after adding 1,6-hexanediamine, so we added a small amount of dilute sulphuric acid instead of NaOH solution such that the gel pH value was the same as the case of [BMIm]Br. The ionic liquid SDA with 2, 6, 8, 10, 12 and 14 carbons in alkyl-tethered imidazolium was also investigated other than [BMIm]Br. The base-hydrolysis sample was prepared following the same procedure, except that the silicate precursor was prepared by catalytically hydrolyzing TEOS in NaOH medium (instead of acidic solution) at pH = 10. In addition to DGC, hydrothermal synthesis of ZSM-22 with [BMIm]Br as SDA was also tried, where the aged gel was directly transferred into a Teflon-lined stainless steel autoclave and crystallized statically at 443 K for 2 days.
Characterization
The crystalline structure of the prepared samples was characterized by X-ray diffraction analysis (XRD) with a SmartLab diffractometer from Rigaku equipped with a 9 kW rotating anode Cu source at 45 kV and 200 mA, from 5° to 50° with a scan rate of 0.2° s−1. The relative crystallinities of the ZX-Y series were calculated roughly from variation of the peaks area in the XRD patterns, assuming crystallinity of 100% for the as-calcined Z35-19 sample. Morphologies of the samples were tested with a field-emission scanning electron microscope (SEM) instrument of Hitachi S-4800. The SiO2/Al2O3 molar ratio of the final solid was measured using ADVANT’XP X-ray fluorescence spectrometer (Thermo Fisher Scientific). Specific surface area and pore volume of the calcined sample were obtained from N2-adsorption–desorption isotherms using multipoint BET and t-plot methods. The samples were outgassed at 573 K for 3 h prior to the measurements. Isotherms were obtained at liquid nitrogen temperature with a BELSORP-MAX analyzer. FT-IR spectra were recorded on a Nicolet iS10 FT-IR instrument (KBr disks) in the 4000–500 cm−1 region. The morphology and microstructure of pure-silica ZSM-22 were determined by transmission electron microscopy (TEM) using a JEM-2100 F. Thermogravimetric (TG) analysis was carried out with an STA 409 instrument in dry air at a heating rate of 10 °C min−1. The 29Si MAS NMR spectrum was recorded on a Bruker Avance 400 D multinuclear solid-state magnetic resonance spectrometer. Solid-state 13C and 1H spin-echo pulse NMR spectra were recorded with a magnetic field of 9.4 T.Results and discussion
DGC synthesis of pure-silica ZSM-22
Fig. 1A shows the XRD patterns of as-synthesized samples ZX-Y series obtained with dry gels containing different amounts of [BMIm]Br and Na2O. All samples gave the high-resolution peaks characterized for the TON-topologized zeolite without any peaks attributable to cristobalite or ZSM-5 that usually jointly occur in static hydrothermal synthesis of ZSM-22.13 After calcination for removal of the SDA, the XRD patterns of all samples exhibit similar peaks to as-synthesized ones with enhanced intensities of some lines, especially the peak assigned to the 110 crystal plane (Fig. 1B), implying the increase of crystallinity and long-range order of the structure as a consequence of reduced electron density in the channel due to elimination of molecules trapped within the channels.24 Moreover, no additional peak assigned to other zeolite phases was detected in the XRD patterns of the calcined samples, suggesting that the DGC method using IL as the SDA is useful for the rapid synthesis of pure-silica ZSM-22.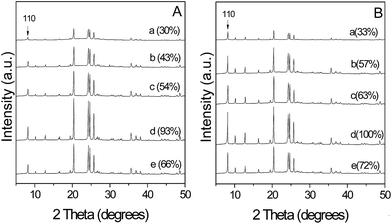 | ||
| Fig. 1 XRD patterns of (A) as-synthesized pure-silica ZSM-22 and (B) as-calcined pure-silica ZSM-22: (a) Z15-15, (b) Z15-19, (c) Z25-19, (d) Z35-19, and (e) Z45-19. | ||
In the synthesis, it was found that concentrations of [BMIm]Br and Na2O exerted significant influences on the crystallinity of pure-silica ZSM-22. In the case of the low concentration of [BMIm]Br and Na2O (x = 0.15 and y = 0.15), the relative crystallinity of the obtained sample Z15-15 was 33% (curve a, Fig. 1B). Keeping the IL concentration constant and increasing the Na2O amount to y = 0.19, the obtained Z15-19 sample presented enhanced diffraction intensities (curve b, Fig. 1B), showing a relative crystallinity of 57%. The impurity phase of magadiite emerged when increasing the Na2O amount to y = 0.22 (curve a, Fig. S2†) and became dominant at higher Na2O concentration of y = 0.24 (curve b, Fig. S2†), indicating that a moderate Na2O amount favored high crystallinity. Fixing the Na2O amount of y = 0.19 while raising the IL amount up to x = 0.35, relative crystallinities of obtained ZX-19 series were increased from 57% to 100% (curves b–d, Fig. 1B), suggesting that a higher concentration of IL favored better crystallinity. Further increasing the IL to x = 0.45, the synthesized Z45-19 showed a relative crystallinity of 72% (curve e, Fig. 1B). The amount of structure-directing agent is closely related to the colloidal properties that influence zeolitic crystal growth in the crystallization process.25 It is suggested in this work that the large amount of IL in the initial gel is not beneficial to the drying process while the water in the undried gel is disadvantageous for the DGC process.26 Therefore, suitable concentration of the IL is important for fabrication of highly crystallized pure-silica ZSM-22.
Synthesis of the pure phase of highly crystallized silica-ZSM-22 is a complicated process affected by various parameters. The positive structure-directing effect of imidazole-based ILs for synthesizing pentasil zeolite was mentioned previously.18 In our synthesis route, the effect of IL [BMIm]Br was investigated by manipulating an SDA-free synthesis procedure. The XRD pattern (curve a, Fig. 2A) shows that the sample synthesized in the absence of [BMIm]Br exhibited the phase of magadiite rather than ZSM-22, suggesting that [BMIm]Br should have acted as SDA in formation of the TON structure. Synthesis of pure-silica ZSM-22 with other SDA was also conducted. Considering that the traditional SDAs in hydrothermal synthesis of ZSM-22 are linear amine salts polyamine and vinyl-pyridine,27 we chose the commonly used 1,6-hexanediamine (HDA) as the structure-directing agent instead of [BMIm]Br in the present DGC route. Following the same procedure, only amorphous phase forms (curve b, Fig. 2A), indicating that [BMIm]Br played an irreplaceable structure-directing role. Actually, only one report has been published for the vapor phase transport synthesis of Al-ZSM-22, in which the mixed vapor phase of diethylamide (Et2N) and water was employed.28 Resulting materials were mostly either cristobalite or quartz together with ZSM-22 zeolite, and the crystallization of pure-phase ZSM-22 needed 7–12 days at 443 K. On the contrary, the present [BMIm]Br IL-directed DGC synthesis favoured the nucleation of ZSM-22 zeolite and promoted rapid crystallization.
For comparison, hydrothermal synthesis of pure-silica ZSM-22 was conducted at 443 K for 2 days after microwave aging of the gel with the same composition as Z35-19. The XRD pattern of the obtained material demonstrated weak peaks of ZSM-22 mixed with magadiite (curve c, Fig. 2A), suggesting that the DGC method also contributes rapid formation of the pure phase of all-silica TON structure. Taking together the fact that the ionic liquid-free dry gel conversion cannot form pure-silica ZSM-22 (curve a, Fig. 2A), both ionic liquid and dry-gel conversion procedures are apparently indispensible for successful rapid synthesis of pure-silica ZSM-22.
The nature of silica precursors plays an important role in the formation of final zeolite phase. Dissolution of silica precursors is more important, through which silicate intermediates are formed followed by the onset of nucleation and crystallization.29 When TEOS was hydrolyzed in basic rather than acidic condition, the obtained pure-silica ZSM-22 sample presented only very weak diffraction intensities (curve d, Fig. 2A), reflecting low crystallinity. In fact, TEOS can be catalytically hydrolyzed at acidic or basic conditions, causing different nucleation and growth rates of the silica precursors. In the acid-catalyzed system, the polymerization rate is more rapid than the hydrolysis rate. After the hydrolytic polymerization, a continuous linear three-dimensional cross-linked network forms, leading to small cage-like units. In contrast, the basic hydrolysis system causes a slower polymerization rate than hydrolysis, which gives rise to short-chain cross-linked larger precursor particles. The resultant different primary structures are largely relative to nucleation rates in these systems.30 Therefore, the acidic condition for preparing silica precursor is crucial for the present DGC method.
To study the influence of the alkyl chain length on the structure-directing effect of alkylimidazolium IL, alkyl chains with 2, 6, 8, 10, 12 and 14 carbon atoms were tethered onto the imidazolium ring for DGC synthesis of ZSM-22 instead of the lead SDA ([BMIm]Br). Fig. 2B shows the XRD patterns of the pure-silica ZSM-22 samples using that series of IL SDAs. When using 1-ethyl-3-methylimidazolium bromide as the SDA, the obtained sample still gave diffraction peaks attributed to the pure-phase TON structure (curve a, Fig. 2B), similar to Z35-19 directed by [BMIm]Br but with the relative crystallinities of 63%. In the case of longer alkyl chains, 1-hexyl-3-methylimidazolium bromide produced a mixture of TON- and MFI-type frameworks (curve b, Fig. 2B), and the further increase of the carbon chains up to 8, 10, 12 and 14 caused no ZSM-22 structure but magadiite or amorphous phases (curves c–f, Fig. 2B). Therefore, the successful synthesis of pure-silica ZSM-22 by DGC route depends partially on the alkyl chain length of alkylimidazolium IL. Moreover, the calculated channel size along the 10-member ring pores in the per unit cell of ZSM-22 was about 1.08 ± 0.04 nm.27 The molecular size of [BMIm]Br was about 1 nm along the alkyl chain direction,18,31 well matching the channel size of ZSM-22. In contrast, the length of 1-hexyl-3-methylimidazolium (∼1.308 nm based on per C–C length 0.154 nm) was larger than the channel size of ZSM-22, let alone the other longer alkylimidazoliums with alkyl carbon chains of 8, 10, 12 and 14, accounting for the unsuccessful synthesis of pure phase silica-ZSM-22. The most effective SDA in the ionic liquid-directed DGC synthesis of TON zeolites was found to be 1-butyl-3-methylimidazolium bromide.
The influence of the microwave aging process was investigated by the control experiment with traditional heating aging. As shown in Fig. 3, the as-synthesized pure-silica ZSM-22 were obtained with heating aging by adjusting aging temperature and time, but crystallinities were low. Moreover, the intensity of XRD peaks decreased and the quartz phase occurred after calcination, suggesting that the heat aging is not suitable to obtain stable ZSM-22. Therefore, microwave aging played an important role in our rapid synthetic route, mostly due to the promoted nucleation and growth of preliminary zeolitic units. Influences of water content in the dry gel and crystallization temperature were also studied, in which it was interesting to observe that another pure-silica zeolite ZSM-48 can be synthesized through the present IL-directed DGC method by adjusting the water content in dry gel and the temperature for crystallization (Fig. S3†), although crystallinity was not high as yet. Such a phenomenon suggested the possibility that other zeolite phases can be also be achieved using our method.
 | ||
| Fig. 3 XRD patterns for as-synthesized (a and c) and as-calcined samples (b and d), obtained through heat aging at 60 °C, 5 h (a and b) and 25 °C, 24 h (c and d). | ||
Textural properties of synthesized pure-silica ZSM-22
Fig. 4A and B show the SEM images of selected pure-silica ZSM-22 sample Z35-19, exhibiting the morphology of an aggregated irregular micron-size prism stacked by thin sheets. The traditional morphology of hydrothermally synthesized ZSM-22 is smaller-sized needle-like crystals. Alfaro et al.32 reached a similar conclusion by comparing the DGC-synthesized silicalite-1 zeolite and that through hydrothermal synthesis. The concentration of raw materials in a hydrothermal system is low because of the large amount of water. The low concentration inhibits the adjacent and aggregation of nuclei during crystal growth, usually causing small crystals. In DGC, a large number of nuclei aggregate because of the limited amount of water, which results in the aggregated morphology with significantly larger sizes. Fig. 4C and D describe the TEM images and selected-area electron diffraction pattern for Z35-19. A clear and well-defined prism plus the ordered microporous channels can be observed, with the selected-area electron diffraction further proving the crystal structure. | ||
| Fig. 4 (A and B) SEM and (C and D) TEM images of as-calcined Z35-19 sample. The inset in the TEM image is the simultaneously recorded SAED pattern from the particle. | ||
Fig. 5A shows the nitrogen adsorption–desorption isotherm for the as-calcined Z35-19 sample. The isotherm is type I, according to the typical microporous structure. There is a high nitrogen uptake at low relative pressures (P/P0 < 0.1), with the BET surface area of 248 m2 g−1 and pore volume of 0.14 cm3 g−1. The as-calcined Z35-19 is further studied by 29Si NMR spectroscopy. As shown in Fig. 5B, there are strong signals at ∼−110, −112 and −113 ppm, indicating that most of the silicon atoms are in four-connected sites (Q4) as expected for a framework silicate. The only weak peak at ∼−100 ppm suggests the presence of Q3 sites in small quantity, indicative of structural defects. The above characterizations present a well-defined zeolitic structure for Z35-19, validating that our DGC method is able to prepare highly crystallized pure-silica ZSM-22.
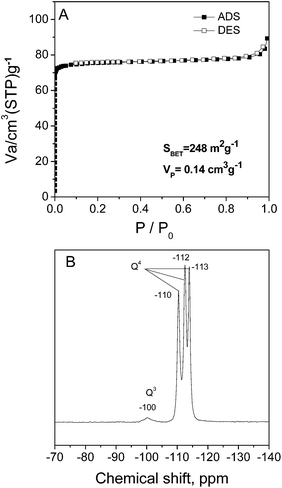 | ||
| Fig. 5 (A) N2 adsorption–desorption isotherm and (B) 29Si MAS NMR spectrum of as-calcined Z35-19 sample. | ||
DGC synthesis of Al-ZSM-22
The ZSM-22 zeolite synthesized above is in the pure-silica state. Indeed, experimenting with Al-containing gels in the subsequent DGC process was quite valuable. Fig. 6 shows the XRD patterns of the as-synthesized and as-calcined Al-containing ZSM-22 samples with SiO2/Al2O3 molar ratios of 200, 150, 100 and 50. As the aluminum amount was raised, the peak intensity decreased, reflecting the decline of crystallinity. When SiO2/Al2O3 was reduced to 50, there was no zeolite phase observed (curve d, Fig. 6A). After calcination, the samples with SiO2/Al2O3 of 200 and 150 presented similar XRD patterns to the as-synthesized parents but with strengthened peak intensities, while the sample with SiO2/Al2O3 of 100 displayed an additional peak at 21.7°, indicating the occurrence of an impurity phase upon calcination. The SiO2/Al2O3 molar ratios of the final zeolite products Al-ZSM-22 were measured by XRF analysis and summarized in Table 1. The results indicated that the SiO2/Al2O3 ratios of the obtained Al-ZSM-22 were slightly lower than those of their parent gels. Adding aluminum in the synthesis causes a complex interaction among silica precursor, aluminum source, Na+ cation and ionic liquid. Zones18 reported an inhibitory effect of aluminum amount on zeolite crystallization referring to the synthesis of pentasil zeolites in the presence of quaternary imidazole compounds. In order to obtain the highly crystallized Al-ZSM-22 in a wide range of SiO2/Al2O3 ratios, further adjusting of the dry-gel composition and crystallization condition may be essential. | ||
| Fig. 6 XRD patterns of (A) as-synthesized and (B) as-calcined Al-ZSM-22: (a) Z35-19-200, (b) Z35-19-150, (c) Z35-19-100, and (d) Z35-19-50. | ||
| Sample | SiO2/Al2O3 (mol mol−1) | |
|---|---|---|
| Gel mixture | As-calcined Al-ZSM-22 | |
| Z35-19-200 | 200 | 152 |
| Z35-19-150 | 150 | 123 |
| Z35-19-100 | 100 | 93 |
Preliminary understanding of structure-directing effect of ionic liquid
FT-IR, TG, 1H and 13C NMR spectra were used to characterize the dry gel and the resulting pure-silica ZSM-22 sample Z35-19, in an attempt to further understand the structure-directing role of [BMIm]Br in the DGC process. Fig. 7A shows the FT-IR spectra of the dry-gel, as-synthesized and as-calcined Z35-19 sample. The dry gel showed vibrations for the BMIm cation in ranges of 1100–1600 and 2700–3200 cm−1 (curve a, Fig. 7A). The bands at 1572 and 1168 cm−1 were attributed to the imidazole C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N stretching vibrations. The C–H bands for the imidazolium ring appeared at 3144 and 3081 cm−1. Moreover, the bands at 2960, 2935 and 2878 cm−1 were attributed to aliphatic C–H bending vibrations.33 The previous result indicated that the ionic liquid was well preserved in dry gel, that is, it had not decomposed throughout the acid-hydrolysis, subsequent alkaline aging, and final drying processes. Even when the crystallization was complete, abovementioned (albeit weak) partial bands were observed, which was attributed to the existing ionic liquid in the as-synthesized ZSM-22 (curve b, Fig. 7A). All BMIm cation peaks disappeared after calcination and the peak intensities of TON framework become stronger at 552, 641, 783, 811 and 1096 cm−1 (curve c, Fig. 7A), reflecting removal of the SDA with well-defined retaining of the zeolite framework. Fig. 7B displays the TG curves of the dry-gel and as-synthesized product of Z35-19. The small weight loss below 200 °C was attributed to physically adsorbed water in both samples. At higher temperatures, the dry gel presented a large weight loss of about 33% ranging from 220 to 370 °C, attributable to decomposition of [BMIm]Br. The decomposition temperature of the gel-involved [BMIm]Br was similar to the free IL, reflecting a weak interaction between the SDA and silica precursor. In contrast, the as-synthesized Z35-19 displayed a slower weight loss up to 420 °C, and the weight loss in the range of ∼200–420 °C was related to water removal in zeolitic internal pores. Dramatic weight loss of 6.2% was observed over 420 °C due to decomposition of [BMIm]Br. The 6.2% weight loss was much lower than that for the dry gel. Such phenomena indicated that the SDA amount in the final zeolite was much lower than that in the dry gel. This is why the as-synthesized Z35-19 sample presented weak peaks in the IR spectrum. Calculated from the weight loss (6.2%) and the molecular weight of [BMIm]Br (219.12 g mol−1), there was about 1.7 × 1020 [BMIm]Br molecules per gram of zeolite Z35-19. Owing to the confined space, the [BMIm]Br molecules were located in the zeolite channel via an end-by-end mode. Therefore, the occupied space of one [BMIm]Br molecule in the Z35-19 channel was about 8.2 × 10−22 cm3. Next, the total occupied space of all [BMIm]Br molecules in the Z35-19 channel was about 0.138 cm3 g−1, in accordance with the pore volume of Z35-19 (0.14 cm3 g−1), suggesting the complete filling of zeolite pores with [BMIm]Br (Fig. 8b and c) just like the role that 1,6-hexanediamine played in hydrothermal synthesis of ZSM-22.27 Such phenomenon further explains why the [BMIm]Br exerted a clear structure-directing effect for the synthesis of TON zeolite. Moreover, the decomposition temperature of the IL was much higher for the as-synthesized sample than the gel, mainly because the IL SDA had been encapsulated in the zeolite channels (schematically illustrated in Fig. 8c). This result provided an important clue for understanding the IL structure-directing effect for the DGC synthesis of pure-silica ZSM-22.
N and C–N stretching vibrations. The C–H bands for the imidazolium ring appeared at 3144 and 3081 cm−1. Moreover, the bands at 2960, 2935 and 2878 cm−1 were attributed to aliphatic C–H bending vibrations.33 The previous result indicated that the ionic liquid was well preserved in dry gel, that is, it had not decomposed throughout the acid-hydrolysis, subsequent alkaline aging, and final drying processes. Even when the crystallization was complete, abovementioned (albeit weak) partial bands were observed, which was attributed to the existing ionic liquid in the as-synthesized ZSM-22 (curve b, Fig. 7A). All BMIm cation peaks disappeared after calcination and the peak intensities of TON framework become stronger at 552, 641, 783, 811 and 1096 cm−1 (curve c, Fig. 7A), reflecting removal of the SDA with well-defined retaining of the zeolite framework. Fig. 7B displays the TG curves of the dry-gel and as-synthesized product of Z35-19. The small weight loss below 200 °C was attributed to physically adsorbed water in both samples. At higher temperatures, the dry gel presented a large weight loss of about 33% ranging from 220 to 370 °C, attributable to decomposition of [BMIm]Br. The decomposition temperature of the gel-involved [BMIm]Br was similar to the free IL, reflecting a weak interaction between the SDA and silica precursor. In contrast, the as-synthesized Z35-19 displayed a slower weight loss up to 420 °C, and the weight loss in the range of ∼200–420 °C was related to water removal in zeolitic internal pores. Dramatic weight loss of 6.2% was observed over 420 °C due to decomposition of [BMIm]Br. The 6.2% weight loss was much lower than that for the dry gel. Such phenomena indicated that the SDA amount in the final zeolite was much lower than that in the dry gel. This is why the as-synthesized Z35-19 sample presented weak peaks in the IR spectrum. Calculated from the weight loss (6.2%) and the molecular weight of [BMIm]Br (219.12 g mol−1), there was about 1.7 × 1020 [BMIm]Br molecules per gram of zeolite Z35-19. Owing to the confined space, the [BMIm]Br molecules were located in the zeolite channel via an end-by-end mode. Therefore, the occupied space of one [BMIm]Br molecule in the Z35-19 channel was about 8.2 × 10−22 cm3. Next, the total occupied space of all [BMIm]Br molecules in the Z35-19 channel was about 0.138 cm3 g−1, in accordance with the pore volume of Z35-19 (0.14 cm3 g−1), suggesting the complete filling of zeolite pores with [BMIm]Br (Fig. 8b and c) just like the role that 1,6-hexanediamine played in hydrothermal synthesis of ZSM-22.27 Such phenomenon further explains why the [BMIm]Br exerted a clear structure-directing effect for the synthesis of TON zeolite. Moreover, the decomposition temperature of the IL was much higher for the as-synthesized sample than the gel, mainly because the IL SDA had been encapsulated in the zeolite channels (schematically illustrated in Fig. 8c). This result provided an important clue for understanding the IL structure-directing effect for the DGC synthesis of pure-silica ZSM-22.
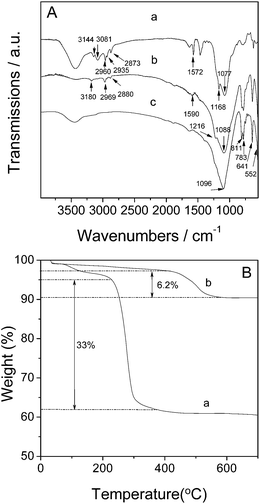 | ||
| Fig. 7 (A) FT-IR spectra of (a) dry gel, (b) as-synthesized, and (c) as-calcined Z35-19; (B) TG curves of (a) dry gel and (b) as-synthesized Z35-19. | ||
Fig. 9A shows the 1H NMR spectra of the dry-gel, as-synthesized and as-calcined Z35-19 sample. The dry gel shows eight peaks at 0.74, 1.18, 1.80, 4.05, 4.36, 7.92, 8.03 and 9.58 ppm (curve a, Fig. 9A), similar to signals of pure [BMIm]Br,34 further indicating that the IL was preserved in the gel and existed as the free state. Only broad and weak signals appeared for the as-synthesized pure-silica ZSM-22 (curve b, Fig. 9A) because of the low SDA amount. In addition, a shift in signals for the H atoms in the imidazolium rings was observed, suggesting a strong interaction between IL and zeolite framework, as reflected by the IR and TG results above. The H signals attributed to IL disappeared after calcination; instead a signal at ∼5 ppm was observed (curve c, Fig. 9A) as a result of the presence of water in as-calcined pure-silica ZSM-22. The 13C chemical shifts for the dry gel and as-synthesized Z35-19 are shown in Fig. 9B. Peaks attributed to the C atoms belonging to imidazolium ring and aliphatic chain can be clearly seen in dry gel in the ranges of 100–150 and 0–50 ppm,17 respectively (curve a, Fig. 9B). Signals at 14.38, 20.02, 32.56, 37.83, 50.04, 123.28, 124.39 and 137.64 ppm were attributed to the eight carbons of [BMIm]Br (Fig. 8a). After crystallization in DGC synthesis, all these signals were still observed but with slightly shifts and declined intensities (curve b, Fig. 9B) due to lower IL content and the strong interaction between the IL and zeolite framework, in accordance with the results of IR, TG and 1H NMR spectra.
 | ||
| Fig. 9 (A) 1H NMR spectra of (a) dry gel, (b) as-synthesized, and (c) as-calcined Z35-19; (B) 13C MAS NMR spectra of (a) dry gel and (b) as-synthesized Z35-19. | ||
The above analysis demonstrates that the IL [BMIm]Br first existed in the dry gel, and then interacted with the silica precursor and located in the microporous channel to cause the formation of TON framework through a structure-directing effect in our DGC synthetic route.
Conclusions
A rapid dry-gel conversion synthetic route was developed to synthesize pure-silica ZSM-22 zeolite with acid-catalyzed hydrolysis of TEOS for preparing the dry gel and using ionic liquid [BMIm]Br as the structure-directing agent. The ionic liquid played the structure-directing role in the synthesis and the dry-gel conversion method contributed to fast crystallization. The crystallization process was much shorter than the conventional hydrothermal process, thereby promoting the potential to prepare functional pure-silica TON topology zeolites such as zeolite membrane or aluminium-free heteroatom zeolites.Acknowledgements
The authors thank the National Natural Science Foundation of China (no. 21136005, 21303038 and 21476109), Jiangsu Province Science Foundation for Youths (no. BK20130921) and the Specialized Research Fund for the Doctoral Program in Higher Education (no. 20133221120002).Notes and references
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS PubMed; M. E. Davis, Nature, 2002, 417, 813 CrossRef PubMed; Y. Tao, H. Kanoh, L. Abrams and K. Kaneko, Chem. Rev., 2006, 106, 896 CrossRef PubMed.
- S. Inagaki, Y. Tsuboi, Y. Nishita, T. Syahylah, T. Wakihara and Y. Kubota, Chem.–Eur. J., 2013, 19, 7780 CrossRef CAS PubMed; N. Sapawe, A. A. Jalil, S. Triwahyono, M. I. A. Shah, R. Jusoh, N. F. M. Salleh, B. H. Hameed and A. H. Karim, Chem. Eng. J., 2013, 229, 388 CrossRef PubMed.
- D. S. Wragg, R. Morris, A. W. Burton, S. I. Zones, K. Ong and G. Lee, Chem. Mater., 2007, 19, 3924 CrossRef CAS.
- J. Stelzer, M. Paulus, M. Hunger and J. Weitkamp, Microporous Mesoporous Mater., 1998, 22, 1 CrossRef CAS.
- E. M. Flanigen, J. M. Bennett, R. W. Grose, J. P. Cohen, R. L. Patton and R. M. Kirchner, Nature, 1978, 271, 512 CrossRef CAS.
- J. C. Waal, M. S. Rigutto and H. Bekkum, J. Chem. Soc., Chem. Commun., 1994, 1241 RSC.
- A. Corma, F. Rey, J. Rius, M. J. Sabater and S. Valencia, Nature, 2004, 431, 287 CrossRef CAS PubMed.
- X. Yang, M. A. Camblor, Y. Lee, H. Liu and D. H. Olson, J. Am. Chem. Soc., 2004, 126, 10403 CrossRef CAS PubMed.
- G. T. Kokotailo, J. L. Schlenker, F. G. Dwyer and E. W. Valyocsik, Zeolites, 1985, 5, 349 CrossRef CAS.
- G. Wang, G. Liu, W. Su, X. Li, Z. Jiang, X. Fang, C. Han and C. Li, Appl. Catal., A, 2008, 335, 20 CrossRef CAS PubMed; J. F. Denayer, G. V. Baron, G. Vanbutsele, P. A. Jacobs and J. A. Martens, Chem. Eng. Sci., 1999, 54, 3553 CrossRef.
- R. Byggningsbacka, L. E. Lindfors and N. Kumar, Ind. Eng. Chem. Res., 1997, 36, 2990 CrossRef CAS; J. I. Villegas, M. Kangas, R. Byggningsbacka, N. Kumar, T. Salmi and D. Y. Murzin, Catal. Today, 2008, 133–135, 762 CrossRef PubMed.
- N. Kumar, L. E. Lindfors and R. Byggningsbacka, Appl. Catal., A, 1996, 139, 189 CrossRef CAS.
- M. A. Asensi, A. Corma, A. Martínez, M. Derewinski, J. Krysciak and S. S. Tamhankar, Appl. Catal., A, 1998, 174, 163 CrossRef CAS; D. Masih, T. Kobayashi and T. Baba, Chem. Commun., 2007, 3303 RSC.
- E. W. Valyocsik, US Pat., 4902406 1990.
- B. Marler, Zeolites, 1987, 7, 393 CrossRef CAS; J. J. Williams, Z. A. D. Lethbridge, G. J. Clarkson, S. E. Ashbrook, K. E. Evans and R. I. Walton, Microporous Mesoporous Mater., 2009, 119, 259 CrossRef PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; K. R. Seddon, Nat. Mater., 2003, 2, 363 CrossRef PubMed.
- R. Kore and R. Srivastava, Catal. Commun., 2012, 18, 11 CrossRef CAS PubMed; R. Kore, B. Satpati and R. Srivastava, Chem.–Eur. J., 2011, 17, 14360 CrossRef PubMed; J. M. M. Blanes, B. M. Szyja, F. Romero-Sarria, M. Á. Centeno, E. J. M. Hensen, J. A. Odriozola and S. Ivanova, Chem.–Eur. J., 2013, 19, 2122 CrossRef PubMed; R. Kore and R. Srivastava, RSC Adv., 2012, 2, 10072 RSC.
- S. I. Zones, Zeolites, 1989, 9, 458 CrossRef CAS.
- S. I. Zones and A. W. Burton, J. Mater. Chem., 2005, 15, 4215 RSC.
- Y. Tian, M. J. Mcpherson, P. S. Wheatley and R. E. Morris, Z. Anorg. Allg. Chem., 2014, 640(6), 1177 CAS.
- S. S. A. Zaidi and S. Rohani, Rev. Chem. Eng., 2005, 21(5), 265 CAS.
- J. Zhou, Z. Hua, J. Zhao, Z. Gao, S. Zeng and J. Shi, J. Mater. Chem., 2010, 20, 6764 RSC.
- Y. Wu, J. Wang, P. Liu, W. Zhang, J. Gu and X. Wang, J. Am. Chem. Soc., 2010, 132, 17989 CrossRef CAS PubMed; J. Gu, Y. Jin, Y. Zhou, M. Zhang, Y. Wu and J. Wang, J. Mater. Chem. A, 2013, 1, 2453 Search PubMed; F. Cao, Y. Wu, J. Gu and J. Wang, Mater. Chem. Phys., 2011, 130, 727 CrossRef PubMed; J. Wang, J. Xie, Y. Zhou and J. Wang, Microporous Mesoporous Mater., 2013, 171, 87 CrossRef PubMed.
- M. Milanesio, G. Artioli, A. F. Gualtieri, L. Palin and C. Lamberti, J. Am. Chem. Soc., 2003, 125, 14549 CrossRef CAS PubMed.
- O. A. Fouad, R. M. Mohamed, M. S. Hassan and I. A. Ibrahim, Catal. Today, 2006, 116, 82 CrossRef CAS PubMed.
- S. P. Naik, A. S. T. Chiang and R. W. Thompson, J. Phys. Chem. B, 2003, 107, 7006 CrossRef CAS.
- S. Ernst, Appl. Catal., 1989, 48, 137 CrossRef CAS.
- S. G. Thoma, D. E. Trudell, F. Bonhomme and T. M. Nenoff, Microporous Mesoporous Mater., 2001, 50, 33 CrossRef CAS.
- S. Mintova and V. Voltchev, Microporous Mesoporous Mater., 2002, 55, 171 CrossRef CAS.
- J. Šefčík and A. V. McCormick, Catal. Today, 1997, 35, 205 CrossRef CAS; B. Tan and S. E. Rankin, J. Phys. Chem. B, 2006, 110, 22353 CrossRef PubMed.
- L. Wang, L. Chang, L. Wei, S. Xu, M. Zeng and S. Pan, J. Mater. Chem., 2011, 21, 15732 RSC.
- S. Alfaro, M. A. Valenzuela and P. Bosch, J. Porous Mater., 2009, 16, 337 CrossRef CAS.
- G. R. Rao, T. Rajkumar and B. Varghese, Solid State Sci., 2009, 11, 36 CrossRef CAS PubMed; Y. Leng, J. Liu, P. Jiang and J. Wang, Catal. Commun., 2013, 40, 84 CrossRef PubMed.
- P. Zhao, M. Zhang, Y. Wu and J. Wang, Ind. Eng. Chem. Res., 2012, 51, 6641 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional XRD patterns and SEM image of samples. See DOI: 10.1039/c4ra07627c |
| This journal is © The Royal Society of Chemistry 2014 |


