The impact of ZIF-8 particle size and heat treatment on CO2/CH4 separation using asymmetric mixed matrix membrane
Abstract
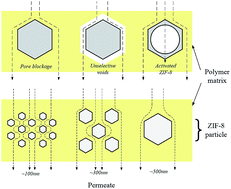
In this study, zeolitic imidazole framework 8 (ZIF-8) particles of different sizes were synthesized in aqueous media by varying the concentration of the base-type additive, triethylamine (TEA). ZIF-8 with particle sizes of ∼134 nm and ∼288 nm with surface areas of 418.44 m2 g−1 and 491.54 m2 g−1 were obtained without altering the crystalline structure. Synthesized ZIF-8 was further heat treated at 100 °C for a minimum of 12 hours, which led to an enhancement of its phase crystallinity and a surface area of 981.1 m2 g−1. Mixed matrix membranes (MMMs) were prepared via the dry–wet phase inversion method by dispersing as-synthesized ZIF-8s, heat-treated ZIF-8s and commercial ZIF-8 (∼493 nm) into a polysulfone (PSf) matrix. The thermal stability and mechanical strength of the membranes showed significant improvement after the incorporation of ZIF-8s. The MMMs were further subjected to the permeation experiments of CO2 and CH4. Although the majority of MMMs showed less selectivity than the neat PSf membrane, the incorporation of heat-treated ZIF-8 of the smallest size, exhibited CO2/CH4 selectivity of 28.5, which is significantly higher than the 19.43 obtained for the neat PSf membrane. Therefore, different ZIF-8 treatment protocols and particle sizes affect the MMMs performance significantly.

 Please wait while we load your content...
Please wait while we load your content...