Pyridoxal hydrazonato molybdenum(VI) complexes: assembly, structure and epoxidation (pre)catalyst testing under solvent-free conditions†
Jana Piskabc,
Biserka Prugovečkia,
Dubravka Matković-Čalogovića,
Tomislav Jednačaka,
Predrag Novaka,
Dominique Agustin *bcd and
Višnja Vrdoljak*a
*bcd and
Višnja Vrdoljak*a
aUniversity of Zagreb, Faculty of Science, Department of Chemistry, Horvatovac, 102a, 10000 Zagreb, Croatia. E-mail: visnja.vrdoljak@chem.pmf.hr; Fax: +385 1 4606 341; Tel: +385 1 4606 353
bUniversité de Toulouse, Institut Universitaire de Technologie Paul Sabatier, Département de Chimie, Av. Georges Pompidou, CS 20258, F-81104 Castres Cedex, France. E-mail: dominique.agustin@iut-tlse3.fr; Fax: +335 63 351 910; Tel: +335 63 621 172
cCNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP 44099, F-31077 Toulouse Cedex 4, France
dUniversité de Toulouse, UPS, INPT, F-31077 Toulouse Cedex 4, France
First published on 14th August 2014
Abstract
Pyridoxal hydrazonato molybdenum(VI) complexes were prepared by the reaction of the corresponding hydrazone (H2L1 = pyridoxal isonicotinic acid hydrazone, H2L2 = pyridoxal benzhydrazone, H2L3 = pyridoxal 4-hydroxy benzhydrazone) and [MoO2(acac)2] under appropriate conditions. The complexes can be classified into three categories: mononuclear [MoO2(L1–3)(MeOH)], polynuclear [MoO2(L1–3)]n and hybrid organic–inorganic compounds with the Lindqvist polyoxomolybdate [MoO2(HL1–3)]2Mo6O19. A unique example of a cationic polymer assembly with Lindqvist anions is reported herein for the first time. The compounds were characterised by elemental, TG and DSC analyses and by spectroscopic (IR, UV-Vis, 1H, 13C NMR) techniques. The crystal and molecular structure of the pyridoxal benzhydrazone H2L2, three mononuclear complexes [MoO2(L1–3)(MeOH)], and the Lindqvist-containing compounds [MoO2(HL2)]2Mo6O19·2MeCN and (H4L1)Mo6O19 were determined by single crystal X-ray diffraction. All complexes were tested as (pre)catalysts for the epoxidation of cyclooctene under solvent-free conditions with the use of aqueous TBHP (TBHP = tert-butylhydroperoxyde) as an oxidant. Optimal results in terms of conversion, selectivity, TOF and TON were obtained at very low (pre)catalyst loadings (0.05% [Mo] vs. substrate). The influence of the Linqvist anion on catalytic performance is discussed.
Introduction
Nowadays, high ecological standards encourage the development of new chemical processes tending to reduce or eliminate the use of solvents.1,2 Recently, we have been involved in the research of highly active and selective molybdenum(VI) catalysts, focusing on catalytic epoxidation using cyclooctene as model substrate under eco-friendly conditions, i.e. using oxidizing agents as aqueous solutions without the addition of extra organic solvents to the reaction media.3–7 Epoxides are interesting target molecules, widely used as raw materials for epoxy resins, paints, surfactants and intermediates in fine chemicals industry.8,9Carefully planned synthetic strategy to obtain oxomolybdenum(VI) compounds can afford interesting architectural motifs.10 The donor ability of the solvent is of crucial importance. With weak donor solvents, pentacoordinated dioxomolybdenum(VI) complex dominantly tend to achieve octahedral coordination by forming O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo⋯Ot interactions11,12 (where Ot is terminal oxygen from the neighboring coordination complex). In specific cases, mononuclear building blocks can form supramolecular architectures [MoO2(L)]x (x = 4, 6 or n) based on O
Mo⋯Ot interactions11,12 (where Ot is terminal oxygen from the neighboring coordination complex). In specific cases, mononuclear building blocks can form supramolecular architectures [MoO2(L)]x (x = 4, 6 or n) based on O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo–N bonds13−15 (where H2L = aroylhydrazone ligand, in which N is nitrogen atom from the neighboring complex). On the contrary, strong donors tend to coordinate to the vacant coordination site affording octahedral coordination. In the “solvent-free quest”, thermally induced solid-state transformations and mechanochemical syntheses also represent important classes of preparative methods.14,16
Mo–N bonds13−15 (where H2L = aroylhydrazone ligand, in which N is nitrogen atom from the neighboring complex). On the contrary, strong donors tend to coordinate to the vacant coordination site affording octahedral coordination. In the “solvent-free quest”, thermally induced solid-state transformations and mechanochemical syntheses also represent important classes of preparative methods.14,16
Relatively few articles report pyridoxal aroylhydrazonato transition metal complexes17–20 and to the best of our knowledge, related molybdenum complexes have not been reported so far. Moreover, rare examples of organic–inorganic hybrid materials based on polyoxometalates (POM) and cationic dioxomolybdenum(VI) complexes [{MoO2(HL)}2Mo6O19] and [MoO2(HL)(D)]2Mo6O19 (D = CH3CN, (CH3)2CO or H2O, H2L = aroyl hydrazone) were described recently.13,21
Although complexes containing the cis-{MoO2}2+ unit are the most widely represented in epoxidation catalysis,22–25 to our knowledge, the literature reports only one catalytic application of the salt containing a Lindqvist POM, [{MoO2(HC(3,5-Me2pz)3)}2(μ2-O)]Mo6O19 (HC(3,5-Me2pz)3 being tris(3,5-dimethyl-1-pyrazolyl)methane).26 The compound was shown to be a fairly stable catalyst for cyclooctene epoxidation with TBHP using different types of organic co-solvents under mild reaction conditions. It has to be pointed out that up to now, no solvent-free epoxidation using the organic–inorganic Lindqvist-type POM has been reported so far.
To investigate the behavior of molybdenum complexes with pyridoxal aroylhydrazonato ligands with or without Lindqvist anions we have targeted the synthetic approach which led to mono and polynuclear dioxomolybdenum(VI) complexes, as well as hybrid organic–inorganic salts of dioxomolybdenum(VI) polynuclear cations and Lindqvist POM. Three different pyridoxal hydrazone based ligands (Scheme 1), H2L1−3 = pyridoxal isonicotinic acid hydrazone, pyridoxal benzhydrazone, pyridoxal 4-hydroxy benzhydrazone were used to achieve this aim. In this research, we studied the synergic effect of the Mo6O192− anion together with a {MoO2}2+ unit in catalysis. As the catalytic studies were performed under (organic) solvent-free conditions, in the presence of water, special attention was devoted to the effect of the latter on the nature of the molybdenum species.
Results and discussion
Dioxomolybdenum(VI) complexes were prepared by the reaction of the corresponding pyridoxal hydrazone H2L1–3 with [MoO2(acac)2] in dry methanol (mononuclear complexes [MoO2(L1,2)(MeOH)] (Ia and IIa) and [MoO2(L3)(MeOH)]·MeOH (IIIa)) or acetonitrile (polynuclear complexes [MoO2(L1,2)]n (I and II) and [MoO2(L1,2)]n·MeCN(III)), Scheme 2. Yellow to orange-red crystals of Ia, IIa and IIIa grew after slow solvent evaporation. Due to lower solubility of the ligands in acetonitrile, longer reaction time (12–16 hours) was required for the preparation of all polynuclear complexes, causing partial ligand decomposition27 and low yields for complexes II (16%) and III (6%). While the mononuclear complex IIa could be transformed to the polynuclear one II by reflux in acetonitrile, we were not able to obtain polynuclear compounds I and III following this procedure, most probably due to low solubility of the mononuclear compounds in acetonitrile. Complex II could also be obtained by exposing the mononuclear complex IIa to acetonitrile vapors for two days. All the polynuclear complexes I, II and III could be transformed into the mononuclear complexes by dissolution in methanol.In all of the neutral dioxomolybdenum(VI) complexes, the ligands are coordinated tridentately in the dianionic fashion (L2−) to the molybdenum centre. In the mononuclear ones Ia–IIIa, the coordination sphere around the molybdenum atom is completed by the coordination of a methanol molecule, whereas in the polynuclear compounds I–III the sixth position is occupied by an oxygen atom from the hydroxyl group of the neighboring complex molecule (Scheme 2).28,29 All complexes exhibit νasym(MoO2) and νsym(MoO2) vibrations characteristic of the cis-{MoO2}2+ core (950–939 cm−1 and 926–912 cm−1 regions for the mononuclear complexes, 950–930 cm−1 and 926–908 cm−1 for the polynuclear ones). A band around 1050 cm−1 present for the mononuclear compounds is assigned to the C–O stretching vibration of the coordinated methanol molecule. Compounds I–III do not show strong bands in the 800–850 cm−1 region (characteristic of the intermolecular Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Mo interaction) excluding the alternative formation of the polynuclear [MoO2L]n complexes linked by oxygen bridges. The characteristic N–H and C
O⋯Mo interaction) excluding the alternative formation of the polynuclear [MoO2L]n complexes linked by oxygen bridges. The characteristic N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration bands at ∼3245 cm−1 and 1680 cm−1, respectively, of H2L1–3 ligands are absent for all neutral complexes and bands at ca. 1600 cm−1 and 1550 cm−1 appear, typical for C=Nimine and C–Ophenolic, respectively. Those bands are indicative of the –N–N=(C–OH)– tautomeric form and coordination to the molybdenum atom through the deprotonated oxygen atom.48,49
O vibration bands at ∼3245 cm−1 and 1680 cm−1, respectively, of H2L1–3 ligands are absent for all neutral complexes and bands at ca. 1600 cm−1 and 1550 cm−1 appear, typical for C=Nimine and C–Ophenolic, respectively. Those bands are indicative of the –N–N=(C–OH)– tautomeric form and coordination to the molybdenum atom through the deprotonated oxygen atom.48,49
When the reactions of [MoO2(acac)2] and H2L1–3 in acetonitrile were carried out in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, the hybrid coordination–inorganic salts [MoO2(HL1−3)]2Mo6O19·2MeCN (1–3) were isolated, involving the cationic polymer assembly [MoO2(HL1−3)]n+ with the Lindqvist anions Mo6O192−. The sixth coordination site of the dioxomolybdenum(VI) complexes occupied by a hydroxyl group from the neighboring pyridoxal moiety provides an extraordinary chain polymerization motif and is the first example of that kind so far. In the IR spectra of compounds 1–3, the presence of the Lindqvist anion is indicated by strong bands at 960 cm−1 and 795 cm−1, attributed to ν(Mo=Ot) and νasym(Mo–Ob),respectively.39 The asymmetric absorption band characteristic for the cis-{MoO2}2+ core was observed around 910 cm−1, while the symmetric one was overlapped by the strong precited Mo
1 molar ratio, the hybrid coordination–inorganic salts [MoO2(HL1−3)]2Mo6O19·2MeCN (1–3) were isolated, involving the cationic polymer assembly [MoO2(HL1−3)]n+ with the Lindqvist anions Mo6O192−. The sixth coordination site of the dioxomolybdenum(VI) complexes occupied by a hydroxyl group from the neighboring pyridoxal moiety provides an extraordinary chain polymerization motif and is the first example of that kind so far. In the IR spectra of compounds 1–3, the presence of the Lindqvist anion is indicated by strong bands at 960 cm−1 and 795 cm−1, attributed to ν(Mo=Ot) and νasym(Mo–Ob),respectively.39 The asymmetric absorption band characteristic for the cis-{MoO2}2+ core was observed around 910 cm−1, while the symmetric one was overlapped by the strong precited Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band of Mo6O192− at 960 cm−1. In the IR spectra of these hybrid compounds the characteristic band for MeCN was observed at 2250 cm−1. The IR spectra (region 1050–550 cm−1) of all the complexes obtained from the ligand H2L1 are presented in the ESI, Fig. S1.†
O band of Mo6O192− at 960 cm−1. In the IR spectra of these hybrid compounds the characteristic band for MeCN was observed at 2250 cm−1. The IR spectra (region 1050–550 cm−1) of all the complexes obtained from the ligand H2L1 are presented in the ESI, Fig. S1.†
It was recently shown that presence of only traces of water was sufficient to transform [MoO2(acac)2]13,21 or Na2MoO4,30 into Mo6O192− containing species. Since our investigations of the catalytic activity of all molybdenum(VI) complexes in olefin epoxidation (vide infra) were carried out in the presence of water, we wondered about the moisture influence on isolated molybdenum complexes. It was found that species 1, 2 and 3 cannot be obtained by hydrolysis of the polynuclear complexes in wet acetonitrile nor by the reaction of (Bu4N)2Mo6O19 with the appropriate mono-(Ia, IIa, IIIa) or polynuclear species (I, II, III) under acidic conditions (acetic acid or HCl). After prolonged standing of 1 in acetone, decomposition occurred and a small amount of (H4L1)Mo6O19·3Me2CO (4) was obtained, containing the doubly protonated hydrazone as counter cation of the Lindqvist moiety. Recrystallization of 2 and 3 from acetone did not produce compounds analogous to 4, although complexes 2 and 3 were completely dissolved.
Thermogravimetric studies
Under pure oxygen atmosphere, the mononuclear complexes Ia and IIa exhibited a first weight loss (in the range 172–248 °C for Ia and 160–190 °C for IIa) corresponding to the release of the coordinated methanol molecule. Upon further heating, decomposition of the complex occurred (in the range 284–471 °C for Ia and 256–440 °C for IIa) leaving MoO3 as residue. For the complex IIIa, loss of the one MeOH molecule starts around 25 °C, followed by the loss of the coordinated MeOH molecule and complex decomposition ending at 436 °C. For the polynuclear complexes, the weight loss was observed in the temperature ranges 272–438 °C (I), 227−430 °C (II) and 240–442 °C (III). In addition, the MeCN molecule in III is lost in the range 60–156 °C. The thermogravimetric analysis of salts 1, 2 and 3 showed decomposition in the temperature ranges 38–386 °C (1), 48–409 °C (2) and 97–412 °C (3). The identity of all MoO3 residues of the thermal analyses was confirmed by PXRD.Solid state transformations with mono- and polynuclear complexes
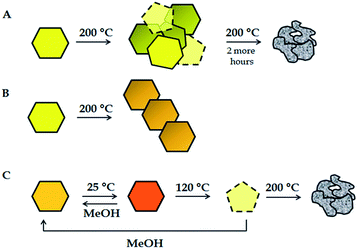
The thermally induced transformations of compounds Ia, IIa and IIIa could be studied in the solid state by X-ray powder diffraction, thermogravimetric studies and IR spectroscopy. The observations are summarized in Fig. 1.The complex Ia shows first structural changes already upon preparation of the sample for PXRD implying that initial grinding process alters the crystal structure. Upon further grinding this phenomenon is even more enhanced (see ESI, Fig. S2†). By heating of Ia at 200 °C, for three hours under pure oxygen, the sample loses crystallinity. However, there is no evidence that a polynuclear compound is formed. Prolonged heating of the sample at 200 °C caused the sample charring. On the other hand, IIa was completely transformed into polynuclear II upon heating at 200 °C for 3 hours (see ESI, Fig. S3†). Initial grinding of IIIa leads to partial loss of the non-coordinated methanol and a color change from orange to red. Nevertheless, the slight change of X-ray diffraction pattern lead to the conclusion that partial solvent loss in IIIa does not significantly alter the crystal structure. Exposure of the dark red product obtained from IIIa to methanol vapors restores the orange color of IIIa. It has to be noted that cristallinity of samples was observed after complete loss of all methanol molecules (non-coordinated and coordinated ones) upon standing for a few weeks at room temperature, turning samples into amorphous materials. Same behavior was observed after heating the complex IIIa at 120 °C for 3 hours, which turned into amorphous material based on the PXRD pattern. A pentacoordinated compound is expected on the bases of TG measurements and IR spectroscopy.14,31 However, polymerization was not achieved even by prolonged heating (2 more hours) of a sample at higher temperatures. The resulting species was an amorphous compound. Heating of IIIa at 200 °C caused the sample charring (see ESI, Fig. S4†).
Influence of mechanochemical treatment was also studied. While microcrystalline samples Ia, IIa, and IIIa turned into amorphous materials when grinded for 30 minutes, exposure of the products to methanol vapors restored crystallinity. This suggests that grinded products are pentacoordinated species, based on IR spectra, different than mononuclear, polynuclear complex and the compounds obtained after heating. The difference occurs in the frequency region of the coordinated methanol molecule, which was previously evidenced in the former group research.14,31
NMR studies
The combined use of one-(1H and APT) and two-dimensional (COSY, HSQC and HMBC) NMR experiments in d6-DMSO made it possible to assign proton and carbon chemical shifts of the ligands and their corresponding molybdenum complexes in solution. The chemical shifts were in accordance with the structures depicted in Scheme S1† and are given in Table S1 (see ESI†). A common feature of the ligands was a significant downfield shift of the NH and OH protons at position 3 and 17, respectively, appearing as separate resonances up to 13 ppm. These resonances are substantially broadened indicating their involvement in hydrogen bonding interactions.Upon complexation to molybdenum, these signals disappeared as a consequence of complex formation. The coordination-induced 13C chemical shift displacements were the largest for the carbonyl carbon nuclei, being shifted downfield by approximately 7 ppm. A surprisingly large downfield shift was observed for the C atom at position 13, amounting to 8.5 ppm for Ia and less for the other two complexes (5.9 ppm for IIa and 5.3 ppm for IIIa). These shifts were the result of electron density redistribution upon ligand coordination to molybdenum. Smaller up- and downfield effects were also observed for other carbon atoms as a consequence of both the coordination induced shifts and the formation of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the –N–N=(C–O)– moiety in the complex instead of a C
N bond of the –N–N=(C–O)– moiety in the complex instead of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the ligand.
O bond in the ligand.
UV-Vis spectroscopic studies
The UV-Vis absorption spectra of compounds (Bu4N)2Mo6O19, 1 and Ia were recorded in acetonitrile in order to test the possible formation of the Lindqvist anion from the mononuclear complex. The lowest energy absorption at 325 nm in the electronic spectrum of (Bu4N)2Mo6O19 was assigned to an O(pπ)–Mo(dπ) LMCT transition, which is bathochromically shifted by 5 nm and maintains nearly identical intensity in the spectrum of 1. This shows that there is no strong interaction between the anionic POM cluster and the cationic molybdenum(VI) complex.32–34 Upon dissolving the mononuclear complexes Ia in acetonitrile, no characteristic band for Mo6O192− was observed in the UV-Vis spectra confirming that [MoO2(L1)(MeOH)] was not hydrolyzed (see ESI, Fig. S5†). The spectral properties of the mononuclear compounds and Mo6O192− salts obtained from the ligands H2L2,3 showed similar behavior as the compounds obtained from H2L1.Crystallographic studies
In the structure of H2L2 (Fig. 2) the pyridoxal ring is not protonated as in the structure of the hydrochloride monohydrate derivative.35 This is supported by the angle at N3 of 117.84(18)° in H2L2 in contrast to 124.5(4)° in the hydrochloride derivative. The C1–O1 and C1–N1 bond lengths and the angles at C1 indicate that the ligand is in the keto form (see ESI, Table S2†). The ligand is not planar and the angle between pyridoxal and the phenyl ring is 23.95(10)°. The packing of H2L2 molecules is characterized by a strong intramolecular hydrogen bond, O2–H2⋯N2, and an extensive intermolecular hydrogen bond framework (see ESI, Table S3, Fig. S6†). The protonated nitrogen atom and the hydroxyl group oxygen atom are involved in hydrogen bonding (N1–H1⋯O3(1 − x, 1 − y, −z)), thus connecting H2L2 molecules into centosymmetrical dimers. O3 serves also as a hydrogen bond donor (O3–H3⋯O1(x, 3/2 − y, 1/2 + z)) forming thus double-layers parallel to (100). There are only van der Waals interactions between the layers.In all mononuclear complexes Ia (see ESI, Fig. S7†), IIa (Fig. 3), IIIa (see ESI, Fig. S8†) and in the hybrid coordination–inorganic salt 2 (Fig. 4), the coordination sphere around the molybdenum atom is a distorted octahedron with the corresponding pyridoxal hydrazone ligand coordinated to the cis-{MoO2}2+ via ONO donor atoms (phenolic-oxygen, azomethine-nitrogen, and enolic-oxygen). The remaining sixth coordination site is occupied by the oxygen atom from the solvent methanol molecule (Ia, IIa and IIIa), or by the oxygen atom from the pyridoxal –CH2OH group from the neighboring molecule (2) thus acting as a linker between the metal centers and forming infinite chains. In Ia, IIa and IIIa, the corresponding ligand is in dianionic form while in 2, the ligand is only singly-deprotonated. The distance from the molybdenum atom to the O atom from methanol or from the pyridoxal –CH2OH group represents the largest bond length within the distorted octahedron (see ESI, Table S4†). Dihedral angles between the two chelate rings formed by complexation of the corresponding ligands to molybdenum, a five-membered (Mo, O1, C1, N1, N2) and a six-membered ring (Mo, O2, C7, C3, C2, N2), amount to 5.03(9)° in Ia, 4.89(9)° in IIa, 10.29(6)° in IIIa and 9.27(17)° in 2.
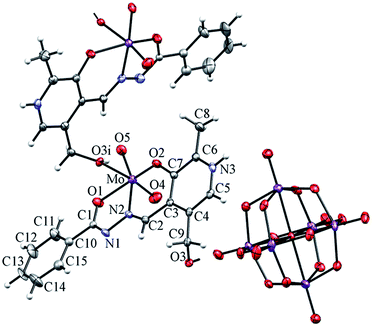 | ||
| Fig. 4 Mercury – POV-Ray drawing of 2. Thermal ellipsoids are at the 50% probability level. i = –x + y, 1 − x, 1/3 + z. Solvent acetonitrile molecules are omitted. | ||
In Ia and IIa, the bond N1–C1 is shortened, whereas C1–O1 is lengthened in comparison to the free ligands H2L1 (reported by J.-P. Souron et al.36) and H2L2, respectively. Similar changes upon coordination were observed in the iron(III) and copper(II) complexes37,38 with H2L1 and dioxouranium(VI) complexes35 with H2L2 (see ESI, Table S3†). There is no structural information for H2L3 but the same trend is expected for the mentioned bond lengths in IIIa.
Since compounds Ia–IIIa have different aromatic substituents on the hydrazone part of the complex, having different hydrogen bond donor/acceptor properties, quite different intermolecular hydrogen bonding and packing was expected. However, the unit cells of Ia and IIa do not differ much and similarities in the powder diffractograms imply similarity in the crystal packing. Indeed, both are characterized by strong hydrogen bonds formed by the O6 atom from the coordinated methanol molecule as a hydrogen bond donor toward the pyridoxal nitrogen atom N3 (2.722(3) Å and 2.716(2) Å in Ia and IIa, respectively) connecting the molecules into endless chains (see ESI, Fig. S9 and S10, Table S3†). Within the chain, there are also π⋯π interactions between the pyridine ring (from pyridoxal) and pyridine in Ia, or phenyl ring in IIa, from a neighboring molecule (see ESI, Table S5†). The difference in the two structures lies in the connection of two chains into double-chains along the b axis through weaker hydrogen bonds involving the O3 atom from the hydroxyl group of the pyridoxal moiety and the N4 pyridine atom (in Ia) or the O6 methanol atom (in IIa).
The asymmetric unit in the crystal structure of IIIa (see ESI, Fig. S8†) consists of the complex molecule and one methanol molecule of crystallization. Atom O6 from the coordinated methanol molecule serves as a hydrogen bond donor to the nitrogen N1 atom (2.7192(19) Å), thus connecting IIIa molecules into centrosymmetrical dimers. Within the dimers, there are also π⋯π interactions between the pyridine ring (from pyridoxal) and 4-hydroxyphenyl group (see ESI, Table S5†). The dimers are interconnected by the hydrogen bond O3–H3⋯O7 (x, y, −1 + z), and also to the methanol molecule of crystallization by hydrogen bonds O7–H7⋯O8(x, −1 + y, z) and O8–H8⋯N3(x, y, 1 + z), connecting the molecules into double-layers parallel to (100) (see ESI, Fig. S11, Table S3†).
The crystal structure of 2 consists of the polynuclear [MoO2(HL2)]n+ cation and the Lindqvist Mo6O192− anion. Only examples of supramolecular Mo(VI) architectures reported in literature are obtained by polymerization through Mo−Npy.12–14 The unique example of the polynuclear cation in 2 is obtained by the coordination of Mo through an O atom from the 3-hydroxy pyridoxal group of a neighboring molecule. In 2, the polynuclear cation forms endless helical chains along the 32 axis, while the anion Mo6O192− lies on a two-fold axis (passing through two molybdenum atoms, the central O atom and two trans O atoms). The chains are interconnected by hydrogen bonds N3–H3⋯O5(1 − x, −x + y, 2/3 − z) (see ESI, Fig. S12, Table S3†). The cation is connected to the POM anion by hydrogen bonds O3–H31⋯O13(1 − y, 1 + x − y, −1/3 + z). The acetonitrile molecules are involved only in weak van der Waals bonding.
The crystal structure of 4 (see ESI, Fig. S13†) consists of {H4L1}2+ cations and Mo6O192− anions and three acetone molecules of crystallization. Crystal structure of H2L1 is known from the literature,36 in the crystal structure of 4 the nitrogen atoms N3 and N4 are protonated which is evident from the value of the C5–N3–C6 angle of 124.7(6)° and C12–N4–C14 angle of 123.2(8)°. The bond lengths C1–O1, C1–N1 and the angles at C1 (see ESI, Table S2†) indicate that the ligand is in the keto form. The ligand is not planar and the angle between pyridoxal and the pyridine ring is 21.3(7)°. The packing of 4 is characterised by a strong intramolecular hydrogen bond between the hydrazone N atom and the 3-hydroxy group from the pyridoxal moiety (O2–H2A⋯N2) and extensive intermolecular hydrogen bonds (see ESI, Table S3†). All three protonated nitrogen atoms (N1, N3 and N4) and oxygen atom O3 (in the pyridoxal CH2OH group) are involved in hydrogen bonding with the acetone molecules (see ESI, Fig. S14†). One acetone molecule acts as bridging interconnecting the {H4L1}2+ cations into chains along [101]. The interwoven chains form a honeycomb network with the anions imbedded into the voids.
Catalytic studies
We have reported the catalytic activity for the solvent-free cyclooctene epoxidation by TBHP of highly active and selective molybdenum (pre)catalysts containing thiosemicarbazonato (ONS) ligands3,4 closely related to the compounds with hydrazonato (ONO) ligands reported here. It was therefore of interest to investigate Ia–IIIa, I–III and 1–3 compounds as (pre)catalysts using the same catalytic process and comparing the results as a function of ligand and type of the complex (mononuclear vs. polynuclear vs. hybrid POM).Catalytic reactions were performed at 80 °C, with cyclooctene as substrate, 0.05% [Mo] loading, and use of aqueous TBHP as oxidant. No organic solvent was added in the reaction mixture. All the complexes were sparingly soluble in cyclooctene and insoluble in water at room temperature, but completely dissolved in the organic phase (coloured yellow) after addition of aqueous TBHP at 80 °C. As previously found, TBHP is mainly transferred in the organic phase (i.e. substrate) under those conditions.7 The reactant and products were only analyzed in the organic layer. Cyclooctene and cyclooctene oxide are not strongly water soluble, therefore the determination of the epoxide selectivity (epoxide formation/cyclooctene conversion) is expected to be exact.
All the results are compiled in Table 1. The cyclooctene conversion for all tested mono- and polynuclear compounds is moderate after 6 h (41–72%), following the order I > IIIa > IIa ∼ II > Ia > III (Fig. 5). In consideration of the very low catalyst loading (0.05% [Mo] vs. substrate), the initial turnover frequencies (TOF20min) are nevertheless notable, the lowest one being 484 h−1 for I and the highest one 1200 h−1 for IIa. The selectivity is moderate to high (67–87%) for all tested compounds, following the order I ∼ II > IIa > IIIa > III > Ia (Table 1).
| Precatalyst | Convb/% | Selc/% | TOF20mind (h−1) | TONe |
|---|---|---|---|---|
| a Reaction conditions: time, 6 h; temperature, 80 °C, catalyst/cyclooctene/TBHP molar ratio: 0.05/100/200 for all compounds except for (Bu4N)2Mo6O19 (0.025/100/200).b For cyclooctene, calculated after 6 h.c Formed epoxide per converted olefin after 6 h.d n(cyclooctene transformed)/n(catalyst)/time at 20 minutes.e n(cyclooctene transformed)/n(catalyst at 6 h).f In case of 1, 2 and 3 and (Bu4N)2Mo6O19 n(catalyst) took into account the “actives species”, i.e. that 1–3 possessed three potential active “sites”. | ||||
| Ia | 41 | 72 | 900 | 820 |
| IIa | 56 | 86 | 1200 | 1080 |
| IIIa | 67 | 75 | 900 | 1340 |
| I | 72 | 87 | 484 | 1440 |
| II | 54 | 87 | 818 | 1120 |
| III | 23 | 73 | 940 | 531 |
| [MoO2(acac)2] | 54 | 82 | 530 | 1300 |
| 1 | 72 | 58 | 44f | 99f |
| 2 | 72 | 53 | 44f | 97f |
| 3 | 76 | 55 | 72f | 105f |
| (Bu4N)2Mo6O19 | 30 | 53 | 42 | 123 |
Among all mononuclear dioxomolybdenum(VI) pre(catalysts), the best epoxide yield after 6 hours was obtained with the complex IIIa and the lowest one with Ia. The ligand nature seems to have a crucial influence. On the basis of the postulated mechanism,6 the pre-catalyst might generate the active pentacoordinated form [MoO2(L)], allowing the TBHP coordination and subsequent activation for the oxygen atom transfer to the outer-sphere olefin, in a mechanism closely related to that proposed by Bartlett for the olefin epoxidation by peracids.39 Complex IIa seems to generate faster the catalytically active pentacoordinated species [MoO2(L2)] much more easily than Ia and IIIa since the initial TOFs is higher for IIa. However, a different trend is shown by the polynuclear compounds I–III, with TOFs of 484 to 940 h−1 show a fast generation of active species in case of III and II and the interesting fact is the deactivation seems to be stronger in the case of complex III. The nature of the ligand (with free phenolic function that could coordinate a free site of the presumed pentacoordinate species) might be the reason of this deactivation.
The pyridoxal hydrazonato complexes reported here afford competitive results (emphasizing solvent-free epoxidation) relative to other molybdenum catalysts reported in the literature. The cyclooctene conversion, the epoxide selectivity, and the TOF and TON values obtained with the pyridoxal hydrazonato compounds are similar to those obtained with the charged pyridoxal thiosemicarbazonato complexes under the same experimental conditions (see ESI, Scheme S2, Table S6†).4 The highest TOF of other representative catalysts used for the epoxidation of cyclooctene, in an organic solvent, by TBHP are 1950 h−1 at 55 °C for [MoO2Cl2(L)2] (H2L = 4,4-bis-methoxycarbonyl-2,2-bipyridine),40 4800 h−1 at 80 °C for cis-[MoO2(phox)2] (H2phox = 2-(2-hydroxyphenyl)oxazoline),41 and 4020 h−1 at 80 °C for [MoO2(L)(MeOH)] (H2L = tridentate Schiff base obtained from a 5-methoxy salicylaldehyde and 1-amino-2-propanol).42 In spite of reported catalytic studies with different types substituted POMs,43,44 only one literature reference reveals the use of molybdenum coordination complex in form of cation and Lindqvist type anion.26 It concerns the cyclooctene epoxidation with TBHP under mild conditions (TBHP in n-decane, 55 °C, in dry DCE, Mo:substrate: TBHP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152), focusing on catalyst recycling and characterization of the recovered catalyst.
152), focusing on catalyst recycling and characterization of the recovered catalyst.
In order to evaluate the possible combined effect of the Mo6O192− and [MoO2(HL1–3)]+ moieties, catalytic investigations were extended to the POM containing salts. In our study, we have selected (Bu4N)2Mo6O19 as reference compound to separately evaluate the activity of the anionic part of compounds 1–3. Under solvent free conditions, at 80 °C with a (Bu4N)2Mo6O19:cyclooctene = 0.025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, using TBHP/H2O as oxidant 15% of cyclooctene conversion was reached after 2.5 h and constant value of 30% was achieved after 4.5 h. The results classified (Bu4N)2Mo6O19 as more active catalyst under solvent-free conditions than operating in organic solvent media.45 As an example, in MeCN/toluene organic medium, H2O2 as oxidant gave 56% conversion after 28 h and TBHP/decane 13% only after 3.5 h using 1
100, using TBHP/H2O as oxidant 15% of cyclooctene conversion was reached after 2.5 h and constant value of 30% was achieved after 4.5 h. The results classified (Bu4N)2Mo6O19 as more active catalyst under solvent-free conditions than operating in organic solvent media.45 As an example, in MeCN/toluene organic medium, H2O2 as oxidant gave 56% conversion after 28 h and TBHP/decane 13% only after 3.5 h using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (Bu4N)2Mo6O19:cyclooctene ratio.
100 (Bu4N)2Mo6O19:cyclooctene ratio.
Under similar experimental conditions with 0.05% [Mo] loading, although the TOF values are low, the presence of the [MoO2(HL1–3)]+ cation resulted in higher cyclooctene conversion than (Bu4N)2Mo6O19 alone (3 > 2 = 1 >> (Bu4N)2Mo6O19), with an average conversion increment of ∼40%, (Fig. 6, Table 1). The selectivity is nearly the same for all tested POM-containing compounds (53–58%, see Table 1). It seems the compounds 1–3 contain two potentially active sites, the [MoO2(HL1–3)]+ cation and the Mo6O192− anion. Nevertheless, comparing all hybrid compounds, the complex 3 has higher TOF value (72 h−1) than 1 = 2 (44 h−1), and kinetic profiles are similar.
In comparison to the mono- and polynuclear compounds, POM compounds 1, 2 and 3 are more active, but less selective towards cyclooctene oxide (Fig. 7). This might be linked to the highest hydrophilicity of POMs that might help the epoxide to be transformed into diol.6
 | ||
| Fig. 7 Comparison of the conversion of cyclooctene (dark bars) and selectivity towards cyclooctene oxide (light bars). Ia–IIIa ([MoO2(L1−3)(MeOH)], I–III ([MoO2(L1−3)]n) and 1–3, ([MoO2(HL1–3)]2Mo6O19·2MeCN). Conversion is calculated for cyclooctene, after 6 h reaction. Selectivity is defined as formed epoxide per converted olefin after 6 h. Exact values are listed in the Table S6 (see ESI†). | ||
Conclusions
A series of novel molybdenum(VI) complexes with hydrazone ligands derived from pyridoxal (mono- and polynuclear complexes as well as hybrid organic–inorganic compounds with the Lindqvist Mo6O192− anion) was prepared and characterized. The thermally induced structure transformations of the complexes were investigated both in solution and in the solid state. The conditions needed for the formation of the hybrid salts were carefully optimized. For the first time the hybrid compound containing a supramolecular chain cation assembly and Lindqvist anion was structurally characterized. The catalytic studies for the solvent-free cyclooctene epoxidation by aqueous TBHP have yielded optimal conversions and selectivities, as well as TOF and TON values. To our knowledge, hybrid catalysts containing both the POM anion and the dioxomolybdenum(VI) complex as cation have been tested here for the first time as (pre)catalysts for epoxidation of olefins under solvent-free conditions. The modulation of reactivity through different Mo/L stochiometries gave different isolated species that exhibited different catalytic activities, very active and selective when Mo/L = 1/1, active but poorly selective when Mo/L = 8/2 and very poor performance when Mo/L = 6/0. Therefore, even if water is present in the catalytic mixture, it can be concluded that the [MoO2L] fragments are sufficiently stable and do not decompose into the Mo6O192− anion under the solvent-free catalytic conditions. In the case of Lindqvist polyoxomolybdates, catalytic activity is doubtlessly related with cationic species.Experimental section
Materials and methods
Pyridoxal hydrochloride, isonicotinic acid hydrazide, benzhydrazide, 4-hydroxy benzhydrazide, ammonium heptamolybdate tetrahydrate, sodium molybdate dihydrate, acetylacetone, tetrabutylammonium bromide, aqueous TBHP (70%), cyclooctene and acetophenone were commercially available. The [MoO2(acac)2] and (Bu4N)2Mo6O19 were prepared as described in the literature.46,47 The ligands were synthesized by literature procedures (see ESI†).48,49 Acetonitrile was dried over phosphorus pentoxide and distilled. Methanol was dried using magnesium turnings and iodine and distilled.The elemental analyses were provided by the Analytical Services Laboratory of the Ruđer Bošković Institute, Zagreb. The thermogravimetric (TG) analyses were performed on a Mettler TG 50 thermobalance using aluminium crucibles in pure oxygen. The differential scanning calorimetry (DSC) measurements were carried out on a Mettler-Toledo DSC823e calorimeter. The results were developed by applying the Mettler STARe 9.01 software. All experiments were recorded in a dynamic atmosphere with a flow rate of 200 cm3 min−1. Infrared spectra were recorded on KBr pellets with a Perkin-Elmer 502 spectrophotometer in the 4400–450 cm−1 region. Electronic absorption spectra were recorded at 25 °C on a Cary 100 UV-Vis Spectrophotometer. 1H and 13C NMR spectra were obtained using a Bruker Avance 600 spectrometer. 1H and 13C chemical shifts of Ia–IIIa are given in Table S1 (see ESI†). The spectra were recorded at 298 K in DMSO-d6 with TMS as internal standard. Experimental details are given in the ESI. For catalytic studies, 1H NMR spectra were obtained using a Bruker Avance DPX-200 spectrometer operating at 200.1 MHz. The catalytic reactions were followed by gas chromatography on an Agilent 6890A chromatograph equipped with FID detector and a DB5-MS capillary column (30 m × 0.32 mm × 0.25 μm). The GC parameters were quantified with authentic samples of the reactants and products. The conversion of cis-cyclooctene and the formation of cyclooctene oxide were calculated from calibration curves (r2 = 0.999) relatively to acetophenone as an internal standard.
Synthesis and spectral properties of obtained complexes
[MoO2(L1)(MeOH)] (Ia). Orange yellow product. Yield: 0.09 g (9%). Anal. calcd for C15H16MoN4O6: C, 40.55; H, 3.63; N, 12.61%; found: C, 40.20; H, 3.32; N, 12.52%. TG: calcd. for MeOH 7.21%, found 6.93%, calcd. for MoO3 32.33%, found 32.10%. IR (KBr disc, cm−1, selected wavelengths): 1603, 1548 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1290 (C–O), 946, 924 (Mo
N), 1290 (C–O), 946, 924 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 271 (16218) and 408 (3162).
O). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 271 (16218) and 408 (3162).
[MoO2(L2)(MeOH)] (IIa). Yellow product. Yield: 0.087 g (16%). Anal. calcd for C16H17MoN3O6: C, 43.35; H, 3.87; N, 9.48%, found: C, 43.10; H, 3.67; N, 9.13%. TG: calcd for MeOH 7.22%, found: 7.11%, calcd. for MoO3 32.47%, found: 32.30%. IR (KBr disc, cm−1, selected wavelengths): 1611, 1539 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1256 (C–O), 950, 926 (Mo
N), 1256 (C–O), 950, 926 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 265 (10233) and 405 (2399).
O). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 265 (10233) and 405 (2399).
[MoO2(L3)(MeOH)]·MeOH (IIIa). Orange red product. Yield: 0.015 g (15%). Anal. calcd for C17H21MoN3O8: C, 41.56; H, 4.31; N, 8.55%, found: C, 41.17; H, 4.12; N, 7.98%. TG: calcd for MoO3 29.29%, found: 28.95%. IR (KBr disc, cm−1, selected wavelengths): 1613, 1559 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1255 (C–O), 939, 912 (Mo
N), 1255 (C–O), 939, 912 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 296 (30200) and 413 (10716).
O). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 296 (30200) and 413 (10716).
[MoO2(L1)]n (I). Dark yellow product. Yield: 0.07 g (83%). Reaction time: 16 hours. Anal. calcd for C14H12MoN4O5: C, 40.79; H, 2.93; N, 13.59%, found: C, 40.76; H, 2.71; N, 13.27%. TG: calcd for MoO3 34.75%, found 34.50%. IR (KBr disc, cm−1, selected wavelengths): 1610, 1559 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1260 (C–O), 930, 908 (Mo
N), 1260 (C–O), 930, 908 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
[MoO2(L2)]n (II). Yellow product. Yield: 0.087 g (16%). Reaction time: 12 hours. Anal. calcd for C16H17MoN3O5: C, 43.81; H, 3.19; N, 10.22%, found: C, 43.84; H, 2.99; N, 10.42%. TG: calcd for MoO3 35.00%, found: 34.63%. IR (KBr disc, cm−1, selected wavelengths): 1608, 1537 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1252 (C–O), 953, 932 (Mo
N), 1252 (C–O), 953, 932 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
[MoO2(L3)]n·MeCN(III). Orange red product. Yield: 0.005 g (6%). Reaction time: 12 hours. Anal calcd for C17H16MoN4O6: C, 43.60; H, 3.44; N, 11.96%, found: C, 43.27; H, 3.11; N, 11.63%. TG: calcd. for CH3CN 8.77%, found 8.87%, calcd for MoO3 30.74%, found: 30.25%. IR (KBr disc, cm−1, selected wavelengths): 1661, 1590 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1259 (C–O), 935, 889 (Mo
N), 1259 (C–O), 935, 889 (Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
[MoO2(HL1)]2Mo6O19·2MeCN (1). Yellow product. Yield: 0.06 g (50%). Anal calcd for C32H32Mo8N10O29: C, 21.49; H, 1.80; N, 7.83%, found: C, 21.17; H, 1.77; N, 7.85. TG: calcd for MoO3 64.41%, found: 64.13%. IR (KBr disc, cm−1, selected wavelengths): 1637, 1569 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1366 (C–O), 960 (MoOt)asym, 912 (MoO2)sym, 793 (MoOb)ayms, 739 (ObMoOt). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 263 (9550) and 329 (5888).
N), 1366 (C–O), 960 (MoOt)asym, 912 (MoO2)sym, 793 (MoOb)ayms, 739 (ObMoOt). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 263 (9550) and 329 (5888).
[MoO2(HL2)]2Mo6O19·2MeCN (2). Yellow product. Yield: 0.087 g (33%). Anal. calcd for C34H34Mo8N8O29: C, 22.86; H, 1.92; N, 6.27%, found: C, 22.53; H, 1.80; N, 5.93%. TG: calcd for MoO3 64.47%, found: 64.12%. IR (KBr disc, cm−1, selected wavelengths): 1605, 1582 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1360 (C–O), 961 (MoOt)asym, (MoO2)asym, 912, 886 (MoO2)sym, 795 (MoOb)asym, 603 (ObMoOt). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 257 (13183), 322 (15488) and 414 (9120).
N), 1360 (C–O), 961 (MoOt)asym, (MoO2)asym, 912, 886 (MoO2)sym, 795 (MoOb)asym, 603 (ObMoOt). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 257 (13183), 322 (15488) and 414 (9120).
[MoO2(HL3)]2Mo6O19·2MeCN (3). Orange product. Yield: 0.032 g (26%). Anal. calcd for C34H34Mo8N8O31: C, 22.46; H, 1.88; N, 6.16%, found: C, 22.12; H, 1.59; N, 5.97%. TG: calcd for MoO3 63.34%, found: 62.98%. IR (KBr disc, cm−1, selected wavelengths): 1612, 1505 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1355 (C–O), 932 (MoOt)asym, 914 (MoO2)asym, 889, 853 (MoO2)sym, 750 (MoOb)asym, 605 (ObMoOt). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 259 (48978), 344 (32359) and 438 (33113).
N), 1355 (C–O), 932 (MoOt)asym, 914 (MoO2)asym, 889, 853 (MoO2)sym, 750 (MoOb)asym, 605 (ObMoOt). UV-Vis (MeCN, λmax/nm (ε/dm3 mol−1 cm−1)): 259 (48978), 344 (32359) and 438 (33113).
General procedure for the epoxidation of cyclooctene by aqueous TBHP
A mixture of cyclooctene (2.76 mL, 20 mmol), acetophenone (internal reference) and Mo (pre)catalyst I–III, Ia–IIIa (0.01 mmol) 1–3 (0.005 mmol), [MoO2(acac)2] (0.005 mmol) and (Bu4N)2Mo6O19 (0.005 mmol) was stirred and heated up to 80 °C before addition of aqueous TBHP (70% w/w, 5.48 mL, 40 mmol). The mixture is initially an emulsion, but two phases become clearly visible as the reaction progresses, a colorless aqueous one and a yellowish organic one. The reaction was monitored for 6 h with withdrawal and analysis of organic phase aliquots (0.1 mL) at required times. Each withdrawn sample was mixed with 2 mL of Et2O, treated with a small quantity of MnO2 and then filtered through silica and analyzed by 1H NMR in CDCl3 or by GC.X-ray Crystallography
| H2L2 | Ia | IIa | IIIa | 2 | 4 | |
|---|---|---|---|---|---|---|
| a R = Σ∣|Fo| − |Fc|∣/Σ|Fo|.b wR = [Σ(Fo2 − Fc2)2/Σw(Fo2)2]1/2.c S = Σ[w(Fo2 − Fc2)2/(Nobs – Nparam)]1/2. | ||||||
| Empirical formula | C15H15N3O3 | C15H16MoN4O6 | C16H17MoN3O6 | C17H21MoN3O8 | C34H34Mo8N8O29 | C23H34Mo6N4O25 |
| Mr | 285.30 | 444.26 | 443.27 | 491.31 | 1786.21 | 1342.18 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Triclinic | Trigonal | Monoclinic |
| Space group | P21/c | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P3221 | Pn |
| Unit cell parameters | ||||||
| a (Å) | 8.537(4) | 7.700(6) | 7.7727(5) | 7.8373(5) | 16.04016(12) | 10.5047(2) |
| b (Å) | 13.573(5) | 8.392(5) | 8.4948(4) | 9.3588(4) | 16.04016(12) | 18.6297(3) |
| c (Å) | 12.290(5) | 13.071(7) | 13.2876(8) | 13.025(3) | 17.79763(16) | 10.7312(2) |
| α (°), β (°), γ (°) | 90, 90.66(4), 90 | 99.59(5), 99.50(6), 93.09(6) | 102.209(5), 100.582(5), 95.244(5) | 92.297(8), 103.058(5), 95.262(5) | 90, 90, 120 | 90, 109.882(2), 90 |
| V (Å3) | 1424.0(10) | 818.5(9) | 835.14(9) | 924.9(2) | 3965.61(8) | 1974.91(7) |
| Z | 4 | 2 | 2 | 2 | 3 | 2 |
| Dcalc (g cm−3) | 1.331 | 1.803 | 1.763 | 1.764 | 2.244 | 2.257 |
| Temperature (K) | 295 | 295 | 295 | 150 | 150 | 295 |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| μ (mm−1) | 0.095 | 0.844 | 0.826 | 0.763 | 1.930 | 1.942 |
| F(000) | 600 | 448 | 448 | 500 | 2586 | 1304 |
| Number of unique data | 2786 | 3523 | 3557 | 3971 | 6372 | 7685 |
| Number of data [Fo ≥ 4σ(Fo)] | 1531 | 3097 | 3107 | 3673 | 6167 | 7199 |
| Number of parameters | 194 | 265 | 248 | 346 | 380 | 564 |
| R1a, [Fo ≥ 4σ(Fo)] | 0.046 | 0.028 | 0.027 | 0.020 | 0.019 | 0.024 |
| wR2b | 0.123 | 0.064 | 0.076 | 0.052 | 0.046 | 0.059 |
| Goodness of fit on F2, Sc | 0.85 | 1.10 | 1.03 | 1.08 | 1.08 | 1.08 |
| Flack parameter | — | — | — | — | −0.038(12) | 0.003(14) |
| Min. and max. electron density (e Å−3) | −0.21, 0.16 | −0.42, 0.44 | −0.43, 1.23 | −0.33, 0.48 | −0.56, 0.55 | −0.83, 1.28 |
Acknowledgements
All authors acknowledge the Ministry of Science, Technology and Sports of the Republic of Croatia (Grant nos. 119-1191342-1082, 119-1193079-1084 and 119-119342-1083) and CNRS for financial support and the University Paul Sabatier (Institut Universitaire Technologique Paul Sabatier) for all research facilities. The fellowship of Jana Pisk was provided by the Ministry of Science, Technology and Sports of the Republic of Croatia, the Croatian Science Foundation (03.01/O-3511-2010) and the French Embassy in Croatia. All authors want to thank Prof. R. Poli for suggestions and advices.Notes and references
- T. Friščić, E. Meštrović, D. Škalec Šamec, B. Kaitner and L. Fábián, Chem.–Eur. J., 2009, 15, 12644 CrossRef PubMed.
- T. Friščić and L. Fábián, CrystEngComm, 2009, 11, 743 RSC.
- J. Pisk, D. Agustin, V. Vrdoljak and R. Poli, Adv. Synth. Catal., 2011, 353, 2910 CrossRef CAS PubMed.
- J. Pisk, B. Prugovečki, D. Matković-Čalogović, R. Poli, D. Agustin and V. Vrdoljak, Polyhedron, 2012, 33, 441 CrossRef CAS PubMed.
- C. Cordelle, D. Agustin, J. C. Daran and R. Poli, Inorg. Chim. Acta, 2010, 364, 144–149 CrossRef CAS PubMed.
- B. Guérin, D. Mesquita-Fernandes, J.-C. Daran, D. Agustin and R. Poli, New J. Chem., 2013, 37, 3466–3475 RSC.
- J. Morlot, N. Uyttebroeck, D. Agustin and R. Poli, ChemCatChem, 2013, 5, 601–611 CrossRef CAS PubMed.
- R. A. Sheldon and J. K. Kochi, in Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed.
- L. C. Hill, in Advances in Oxygenated Processes, ed. A. L. Baumstark, JAI Press Inc., London, 1988, vol. 1 Search PubMed.
- Y.-F. Song, D.-L. Long, C. Ritchie and L. Cronin, Chem. Rev., 2011, 158 Search PubMed.
- B. Zhao, X.-Y. Chen, P. Cheng, B. Ding, D.-Z. Liao, S.-P. Yang and Z.-H. Jiang, J. Coord. Chem., 2005, 467 CrossRef CAS.
- M. Abrantes, T. R. Amarante, M. Antunes, S. Gago, F. A. A. Paz, M. Pillinger, A. A. Valente and I. S. Gonçalves, Inorg. Chem., 2010, 6865 CrossRef CAS PubMed.
- V. Vrdoljak, B. Prugovečki, D. Matković-Čalogović, R. Dreos, P. Siega and C. Tavagnacco, Cryst. Growth Des., 2010, 10, 1373 CAS.
- V. Vrdoljak, B. Prugovečki, D. Matković-Čalogović, J. Pisk, R. Dreos and P. Siega, Cryst. Growth Des., 2011, 11, 1244 CAS.
- V. Vrdoljak, B. Prugovečki, D. Matković-Čalogović, T. Hrenar, R. Dreos and P. Siega, Cryst. Growth Des., 2013, 13, 3773 CAS.
- R. Zboril, M. Mashlan, K. Barcova and M. Vujtek, Hyperfine Interact., 2002, 139–140, 597 CrossRef.
- P. Domiano, A. Musatti, M. Nardell, C. Pelizzi and G. Predieri, Transit. Met. Chem., 1979, 4, 351 CrossRef CAS; T. B. Murphy, N. J. Rose, V. Schomaker and A. Aruffo, Inorg. Chim. Acta, 1985, 108, 183 CrossRef.
- T. R. Rao and G. Singh, Cryst. Res. Technol., 1989, 24, K169 CrossRef CAS PubMed.
- S. Avramovici-Grisaru, S. Sarel, S. Cohen and R. E. Bauminger, Isr. J. Chem., 1985, 25, 288 CrossRef CAS PubMed.
- T. B. Murphy, D. K. Johnson, N. J. Rose, A. Aruffo and V. Schomaker, Inorg. Chim. Acta, 1982, 66, L67 CrossRef CAS.
- V. Vrdoljak, B. Prugovečki, D. Matković-Čalogović and J. Pisk, CrystEngComm, 2011, 13, 4382 RSC.
- J. Zhao, X. Zhou, A. M. Santos, E. Herdtweck, C. C. Romao and F. E. Kühn, Dalton Trans., 2003, 3736 RSC.
- Y. Sui, X. Zeng, X. Fang, X. Fu, Y. Xiao, L. Chen, M. Li and S. Cheng, J. Mol. Catal. A: Chem., 2007, 270, 61 CrossRef CAS PubMed.
- A. Rezaeifard, M. Jafarpour, H. Raissi, M. Alipour and H. Stoeckli Evans, Z. Anorg. Allg. Chem., 2012, 638, 1023 CrossRef CAS PubMed.
- G. Chahboun, J. A. Brito, E. Gómez-Bengoa, M. E. G. Mosquera, T. Cuenca and E. Royo, Eur. J. Inorg. Chem., 2012, 2940 CrossRef CAS PubMed.
- A. C. Gomes, P. Neves, S. Figueiredo, J. A. Fernandes, A. A. Valente, F. A. A. Paz, M. Pillinger, A. D. Lopes and I. S. Gonçalves, J. Mol. Catal., 2013, 370, 64 CrossRef CAS PubMed.
- D. Richardson, L. Wis Vitolo, E. Bakers and J. Webb, BioMetals, 1989, 2, 69 CAS.
- M. B. Ferrari, F. Bisceglie, G. Pelosi, P. Tarasconi, R. Albertini, P. P. Dall'Aglio, S. Pinelli, A. Bergamo and G. Fava, J. Inorg. Biochem., 2004, 98, 301 CrossRef CAS PubMed.
- K. Aoki, N. Hu and H. Yamazaki, Inorg. Chim. Acta, 1991, 186, 253 CrossRef CAS.
- E. C. Alyea, D. Craig, I. Dance, K. Fisher, G. Willett and M. Scudder, CrystEngComm, 2005, 7, 491 RSC.
- K. Užarević, M. Rubčić, I. Đilović, Z. Kokan, D. Matković-Čalogović and M. Cindrić, Cryst. Growth Des., 2009, 9, 5327 Search PubMed.
- Q. Li, P. Wu, Y. Xia, Y. Wei and H. Guo, J. Organomet. Chem., 2006, 691, 1223 CrossRef CAS PubMed.
- Q. Li, L. Zhu, X. Meng, Y. Zhu, J. Hao and Y. Wei, Inorg. Chim. Acta, 2007, 360, 2558 CrossRef CAS PubMed.
- Q. Li, Y. Wei, H. Guo and C.-G. Zhan, Inorg. Chim. Acta, 2008, 361, 2305 CrossRef CAS PubMed.
- D. F. Back, M. A. Ballin and G. M. de Oliveira, J. Mol. Struct., 2009, 935, 151 CrossRef CAS PubMed.
- J.-P. Souron, M. Quarton, F. Robert, A. Lyubachova, A. Cosse-Barbi and J. P. Doucet, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, 51, 2179 CrossRef.
- T. B. Murphy, N. J. Rose, V. Schomaker and A. Aruffo, Inorg. Chim. Acta, 1985, 108, 183 CrossRef CAS.
- T. R. Rao and G. Singht, Cryst. Res. Technol., 1989, 24, K169 CrossRef CAS PubMed.
- P. D. Bartlett, Rec. Chem. Prog., 1950, 11, 47 CAS.
- A. Guenyar, D. Betz, M. Drees, E. Herdtweck and F. E. Kühn, J. Mol. Catal. A: Chem., 2010, 331, 117 CrossRef CAS PubMed.
- M. Bagherzadeh, L. Tahsini, R. Latifi and L. K. Woo, Inorg. Chim. Acta, 2009, 362, 3698 CrossRef CAS PubMed.
- A. Rezaeifard, I. Sheikhshoaie, N. Monadi and M. Alipour, Polyhedron, 2010, 29, 2703 CrossRef CAS PubMed.
- N. Mizuno, K. Yamaguchi and K. Kamata, Coord. Chem. Rev., 2005, 249, 1944 CrossRef CAS PubMed.
- K. Kamata, K. Yonehara, Y. Sumida, K. Yamaguchi, S. Hikichi and N. Mizuno, Science, 2003, 300, 964 CrossRef CAS PubMed.
- C. Bhaumik, E. Manoury, J. C. Daran, P. Sözen-Aktaş, F. Demirhan and R. Poli, J. Organomet. Chem., 2014, 760, 115 CrossRef CAS PubMed.
- G. J.-J. Chen, W. J. McDonald and W. E. Newton, Inorg. Chem., 1976, 15, 2612 CrossRef CAS.
- N. H. Hur, W. G. Klemperer and R.-C. Wang, Inorg. Synth., 1990, 27, 78 Search PubMed.
- M. F. Belicchi, G. F. Gasparri, E. Leporati, C. Pelizzi, P. Tarasconi and G. Tosi, J. Chem. Soc., Dalton Trans., 1986, 2455 RSC.
- M. R. Maurya, S. Argarwal, C. Bader and D. Rehder, Eur. J. Inorg. Chem., 2005, 147 CrossRef CAS PubMed.
- X'Pert Software Suite, Version 1.3e, Panalytical B.V., Almelo, The Netherlands, 2001 Search PubMed.
- Xcalibur CCD system, CRYSALIS Software system CrysAlisPro, Version 1.171.34.44, Oxford Diffraction Ltd., Oxfordshire, UK, 2010 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, M. Towler, J. Van der Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Ligand preparation, IR spectra, PXRD patterns, NMR data, UV-Vis spectra, crystallographic data. CCDC 985497–985502. For ESI and crystallographic data in CIF or other electronic format See DOI: 10.1039/c4ra08179j |
| This journal is © The Royal Society of Chemistry 2014 |