Alkoxy base-mediated transition-metal-free cross-coupling reactions of benzene with aryl halides†
Wei Liu*,
Lige Xu and
Yanlan Bi
Lipid Chemsitry, College of Food Science and Technology, Henan University of Technology, Lianhua Street, Zhengzhou 450001, Henan, P. R. China. E-mail: liuwei307@hotmail.com; Fax: +86-0371-67758022; Tel: +86-0371-67758022
First published on 9th September 2014
Abstract
Mixed alkoxy bases (EtOK/t-BuOK) can efficiently promote the cross-coupling of benzene and aryl iodides at 80 °C in the absence of any amine or phenanthroline ligand. This transition-metal-free direct C–H arylation offers a simple and efficient way to synthesize biaryl compounds in good yields.
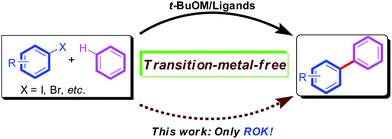
Biaryl compounds are very important subunits which are often found in natural products, pharmaceuticals and organic materials.1 For the past few years, transition metal-catalyzed direct C–H arylation of unactivated arenes (benzene, e.g.) with aryl halides to afford aryl-aryl motifs has been successfully achieved.2 However, the demand for green chemistry inspired organic chemists to develop a transition-metal-free coupling reaction process.3
In 2008, Itami and co-workers first reported a t-BuOK promoted transition-metal-free cross-coupling between nitrogen heterocycles and haloarenes under microwave irradiation.4 In 2010, three research groups independently disclosed transition-metal-free cross-coupling of unactivated arenes and aryl halides involving base-promoted homolytic aromatic substitution (HAS).5 In these processes, a catalytic amount of diamine ligands (phenanthroline or DMEDA)5 could efficiently promote the cross-coupling between aryl iodides/bromides with unactivated arenes (benzene, etc.) under t-butoxides (t-BuOK or t-BuONa) mediated conditions. Since then, considerable attention has been focused on developing new ligands or initiators6 toward this newly methodology. And this transition-metal-free strategy has been also successfully extended to intramolecular arylation for the synthesis of cyclic compounds.7 Other transition-metal-free reaction systems8 have been developed for the cross-coupling between unactivated benzene and aryl iodides, which needed to be conducted at harsh reaction temperature (200 °C)8a or under photo-irradiation conditions.8b–d Very recent effort revealed that t-BuOK alone indeed can promote the intermolecular arylation between benzene and aryl iodides at high reaction temperature (160 °C)9 or the intramolecular arylation for special substrates.7e
However, to the best of our knowledge, no example of alkoxy base (ROK) alone promoted the coupling of benzene and aryl halides at a milder temperature (80 °C) has been reported (Scheme 1). Herein, we report our results on the EtOK/t-BuOK promote direct C–H arylation of unactivated benzene with aryl iodides at 80 °C with good yields.
In continuing our interest on base-promoted direct C–H arylation of unactivated arenes,6g we propose that ROK alone can promote such coupling reactions at mild reaction temperature. We embarked on this research by testing the feasibility of the coupling reaction between 4-iodoanisole (1a) and benzene (2a) at 80 °C (Table 1). In the presence of KOH (3 equiv.), no reaction occurred after 24 h (Table 1, entry 1). Then a set of commercial available alkoxy bases (3 equiv.), such as MeOK, EtOK, t-BuOK and t-AmOK were tested for this aromatic C–H transformation, and no targeted coupling product was observed (Table 1, entries 2–5). To our surprise, the combination of KOH (1 equiv.) and t-BuOK (3 equiv.) led to 6% yield of 4-methoxybiphenyl (3a) and 12% conversion of 1a (Table 1, entry 6) at a much lower temperature (80 °C). We next examined other combinations of mixed alkoxy bases under standard conditions. To our delight, when the combination bases turned to MeOK/t-BuOK (1/3), the substrate 1a were completely consumed and 80% yield of desired product 4-methoxybiphenyl (3a) was obtained (Table 1, entry 7). And 84% yield of 3a was obtained under EtOK/t-BuOK (1/3) conditions (Table 1, entry 8). The use of other combinations (MeOK/t-AmOK and EtOK/t-AmOK) could also provided the desired coupling product 3a in moderate yields (58–70%) (Table 1, entries 9–10). Moreover, the amount of mixed bases EtOK/t-BuOK was investigated and the results showed that decreasing the amount of either EtOK or t-BuOK led to low conversions and low yields (Table 1, entries 11 and 12). It was worth noting that EtOK/t-BuONa could also promote such coupling, albeit in very low yield (17%) (Table 1, entry 13). However, no coupling reaction occurred under EtOK/t-BuOLi or t-BuONa mediated conditions (Table 1, entries 14 and 15). These experiments demonstrated the superiority of the potassium cation for effecting these reactions. And various transition-metal salts showed no effect in the control experiments (Table S1, ESI†).
| Entry | Additives | Base (equiv.) | Conv.b (%) | Yieldb (%) |
|---|---|---|---|---|
| a Standard reactions conditions: 1a (0.5 mmol), benzene (4.0 mL), 80 °C, 24 h, N2.b Calibrated GC yields were reported using hexadecane as the internal standard. | ||||
| 1 | None | KOH (3.0) | <5 | 0 |
| 2 | None | MeOK (3.0) | 7 | 0 |
| 3 | None | EtOK (3.0) | 10 | 0 |
| 4 | None | t-BuOK (3.0) | 5 | 0 |
| 5 | None | t-AmOK (3.0) | 6 | 0 |
| 6 | None | KOH (1.0)/t-BuOK (3.0) | 12 | 6 |
| 7 | None | MeOK (1.0)/t-BuOK (3.0) | >99 | 80 |
| 8 | None | EtOK (1.0)/t-BuOK (3.0) | >99 | 84 |
| 9 | None | MeOK (1.0)/t-AmOK (3.0) | 65 | 58 |
| 10 | None | EtOK (1.0)/t-AmOK (3.0) | 90 | 70 |
| 11 | None | EtOK (0.5)/t-BuOK (3.0) | 60 | 51 |
| 12 | None | EtOK (1.0)/t-BuOK (2.0) | 78 | 61 |
| 13 | None | EtOK (1.0)/t-BuONa (3.0) | 24 | 17 |
| 14 | None | EtOK (1.0)/t-BuOLi (3.0) | 8 | 0 |
| 15 | None | t-BuONa (3.0) | 3 | 0 |
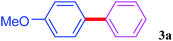
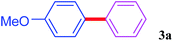
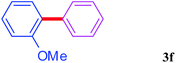
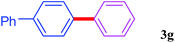
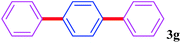
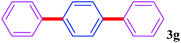
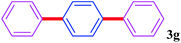
With the optimal conditions in hand, we investigated the direct arylations of unactivated benzene with a range of aryl halides (Table 2). Simple iodobenzene (1b) and other aryl iodides bearing electron-donating or withdrawing groups could be efficiently coupled with benzene to generate the desired products in good yields (46–77%) (Table 2, entries 1–11). Notably, sterically hindered aryl iodides (1e and 1f) were suitable under the same reaction conditions and afforded the aromatic C–H arylated products (3e and 3f) in moderate yields (34% and 42%), respectively (Table 2, entries 7 and 8). 4-FC6H4–I (1i) coupled with benzene smoothly and produced the desired arylated products in 60% yield (Table 2, entry 11). 1,4-Diiodobenzene (1j) underwent double arylation and produced p-terphenyl (3g) in 47% yield (Table 2, entry 12). Interestingly, the use of other dihaloarenes substrates (1k and 1l) all produced the double arylation product p-terphenyl 3g as the major product in 42–43% yields under the standard conditions (Table 2, entries 13 and 14). However, aryl bromides or chlorides such as 4-bromoanisole (1a′) or 4-chloroanisole (1a′′) were less reactive under the identical conditions (Table 2, entries 2 and 3).
| Entry | Aryl halides (1) | Product (3) | Yieldb (%) |
|---|---|---|---|
| a Reactions conditions: 1 (0.5 mmol), EtOK (0.5 mmol), t-BuOK (1.5 mmol), benzene (4.0 mL), 80 °C, 24 h, N2.b Isolated yield based on 1.c 8% yield of 4-bromophenyl was detected.d 10% yield of 4-chlorophenyl was detected. | |||
| 1 |  |
 |
77 |
| 2 | 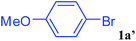 |
 |
21 |
| 3 | 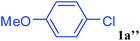 |
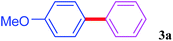 |
<5 |
| 4 | 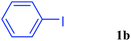 |
 |
75 |
| 5 | 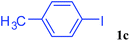 |
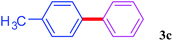 |
80 |
| 6 | 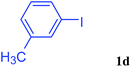 |
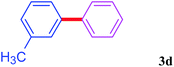 |
73 |
| 7 | 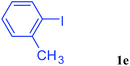 |
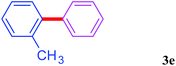 |
34 |
| 8 | 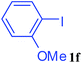 |
 |
42 |
| 9 | 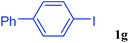 |
 |
65 |
| 10 | 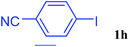 |
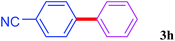 |
46 |
| 11 | 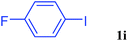 |
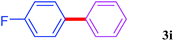 |
60 |
| 12 | 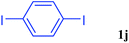 |
 |
47 |
| 13 |  |
 |
43c |
| 14 |  |
 |
42d |
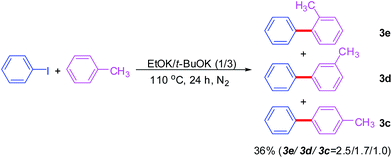
Next, other substituted benzenes, such as toluene, was also examined in this reaction (Scheme 2). And the coupling between toluene and iodobenzene could undergo smoothly at 110 °C and afforded a mixture of regioisomers favoring the ortho isomers. The regioselectivity observed in our system was similar with that in previous reports.5,6 Considering a mixture of regioisomers obtained from the coupling between monosubstituted benzenes and aryl iodides, we did not further tested other substituted benzenes.
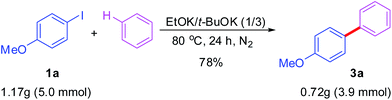
To make our strategy practically viable, the reaction was conducted with 5 mmol of 1a, affording desired coupling product 3a in 78% yield without any further purification (column chromatography, eg.) (Scheme 3).
To gain further insight into the mechanism of such transition-metal-free coupling, we next tested a set of control experiments (Table 3). The coupling reaction could occur in dark conditions with good yield (82%) (Table 3, entry 2), which clearly ruled out the photostimulated SRN1 reactions.8b,10 When the reaction conducted under oxygen atmosphere, nearly no coupling product was observed, which indicated that this reaction was oxygen sensitive (Table 3, entry 3). Moreover, the coupling reaction was almost shut down when 2,2,6,6-tetramethyl-1-piperidinoxyl (TEMPO) or 1,1-diphenylethylene were added as radical inhibitors (Table 3, entries 4 and 5). These results demonstrated that these biaryl coupling involved radical reaction mechanism.5
| Entry | Condition | Conv.b (%) | Yieldb (%) |
|---|---|---|---|
| a Reactions conditions: 1a (0.5 mmol), EtOK (0.5 mmol), t-BuOK (1.5 mmol), benzene (4.0 mL), 80 °C, 24 h.b Calibrated GC yields were reported using hexadecane as the internal standard. | |||
| 1 | Nitrogen atmosphere | >99 | 84 |
| 2 | Dark | >99 | 82 |
| 3 | Oxygen atmosphere | <5 | <5 |
| 4 | TEMPO (1.0 equiv.) | 8 | <5 |
| 5 | 1,1-Diphenylethylene (1.0 equiv.) | 10 | 6 |
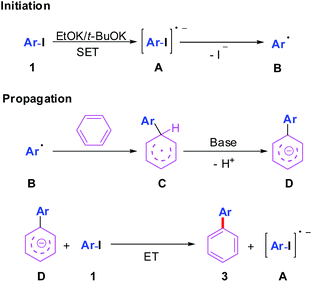
Previous studies5,6 have disclosed that the rate-determining step in such radical coupling was single electron transfer (SET) process in the initiation step to generate aryl iodides radical anion A (Scheme 4). In most cases, ligands were proposed to promote this SET process. Notably, Tuttle and Murphy6l recently envisage that a very small concentration of benzyne could be formed as an electron donor and initiated the SET process in the absence of “additives”. Although the reactions of various substituted aryl iodides with benzene all produced the sole corresponding direct arylation products under our standard conditions (Table 2), the initiation mechanism involving trace amount of benzyne as radical chain reaction initiator was possible.11
Thus, we propose that this radical coupling go through base promoted HAS mechanism (Scheme 4).5d In the initiation step, an aryl iodides radical anion A is generated from 1 through SET process in the presence of EtOK/t-BuOK and converted into an aryl radical B upon dehalogenation. The aryl radical B then react with benzene to form cyclohexadienyl radical C. In the presence of a strong base, the resulting cyclohexadienyl radical C is deprotonated to give biaryl radical anion D. Then a radical chain transfer occurs between D and aryl iodides 1, resulting in the formation of coupling product 3 and the regeneration of radical anion A.
Conclusions
In conclusion, we have developed a mild radical coupling of unactivated benzene with aryl halides to prepare biaryl compounds in the presence of EtOK and t-BuOK at 80 °C. Moreover, double C–H bond arylation was successfully achieved to construct extended π−electron systems. Further studies on the mechanism and the application to other reactions are underway in our laboratory, and will be reported in due course.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21102036), Plan For Scientific Innovation Talent of Henan University of Technology (11CXRC02) and startup fund from HAUT (2010BS042).Notes and references
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (b) O. Daugulis, H. Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (c) G. P. McGlacken and L. M. Bateman, Chem. Soc. Rev., 2009, 38, 2447 RSC; (d) A. W. Lei, W. Liu, C. Liu and M. Chen, Dalton Trans., 2010, 39, 10352 RSC; (e) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed.
- For selected examples on biaryl coupling in the presence of strong bases (t-BuOK), see: (a) K. Fujita, M. Nonogawa and R. Yamaguchi, Chem. Commun., 2004, 1926 RSC; (b) O. Kobayashi, D. Uraguchi and T. Yamakawa, Org. Lett., 2009, 11, 2679 CrossRef CAS PubMed; (c) F. Vallee, J. J. Mousseau and A. B. Charette, J. Am. Chem. Soc., 2010, 132, 1514 CrossRef CAS PubMed; (d) W. Liu, H. Cao and A. W. Lei, Angew. Chem., Int. Ed., 2010, 49, 2004 CrossRef CAS PubMed; (e) H. Li, C.-L. Sun, M. Yu, D.-G. Yu, B.-J. Li and Z.-J. Shi, Chem.–Eur. J., 2011, 17, 3593 CrossRef CAS PubMed; (f) C. T. To, T. L. Chan, B. Z. Li, Y. Y. Hui, T. Y. Kwok, S. Y. Lam and K. S. Chan, Tetrahedron Lett., 2011, 52, 1023 CrossRef CAS PubMed.
- For selected reviews on transition-metal-free coupling reactions, see: (a) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC; (b) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC; (c) T. L. Chan, Y. Wu, P. Y. Choy and F. Y. Kwong, Chem. –Eur. J., 2013, 19, 15802 CrossRef CAS PubMed; (d) R. A. D. Arancon, C. S. K. Lin, C. Vargas and R. Luque, Org. Biomol. Chem., 2014, 12, 10 RSC.
- S. Yanagisawa, K. Ueda, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 4673 CrossRef CAS PubMed.
- (a) C.-L. Sun, H. Li, D.-G. Yu, M. Yu, X. Zhou, X. Y. Lu, K. Huang, S. F. Zheng, B.-J. Li and Z.-J. Shi, Nat. Chem., 2010, 2, 1044 CrossRef CAS PubMed; (b) W. Liu, H. Cao, H. Zhang, H. Zhang, K. H. Chung, C. He, H. B. Wang, F. Y. Kwong and A. W. Lei, J. Am. Chem. Soc., 2010, 132, 16737 CrossRef CAS PubMed; (c) E. Shirakawa, K. Itoh, T. Higashino and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 15537 CrossRef CAS PubMed; (d) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018 CrossRef CAS PubMed.
- (a) Y. T. Qiu, Y. H. Liu, K. Yang, W. K. Hong, Z. Li, Z. Y. Wang, Z. Y. Yao and S. Jiang, Org. Lett., 2011, 13, 3556 CrossRef CAS PubMed; (b) G.-P. Yong, W.-L. She, Y.-M. Zhang and Y.-Z. Li, Chem. Commun., 2011, 47, 11766 RSC; (c) H. Liu, B. Yin, Z. Gao, Y. Li and H. Jiang, Chem. Commun., 2012, 48, 2033 CAS; (d) W.-C. Chen, Y.-C. Hsu, W.-C. Shih, C.-Y. Lee, W.-H. Chuang, Y.-F. Tsai, P. P.-Y. Chen and T.-G. Ong, Chem. Commun., 2012, 48, 6702 RSC; (e) K. Tanimoro, M. Ueno, K. Takeda, M. Kirihata and S. Tanimori, J. Org. Chem., 2012, 77, 7844 CrossRef CAS PubMed; (f) H. Zhao, J. Shen, J. Guo, R. Ye and H. Zeng, Chem. Commun., 2013, 49, 2323 RSC; (g) W. Liu, F. Tian, X. Wang, H. Yu and Y. Bi, Chem. Commun., 2013, 49, 2983 RSC; (h) A. Dewanji, S. Murarka, D. P. Curran and A. Studer, Org. Lett., 2013, 15, 6102 CrossRef CAS PubMed; (i) B. Li, X. Qin, J. You, X. Cong and J. Lan, Org. Biomol. Chem., 2013, 11, 1290 RSC; (j) S. Sharma, M. Kumar, V. Kumar and N. Kumar, Tetrahedron Lett., 2013, 54, 4868 CrossRef CAS PubMed; (k) S. A., X. Liu, H. Li, C. He and Y. Mu, Asian J. Org. Chem., 2013, 2, 857 CrossRef; (l) S. Zhou, G. M. Anderson, B. Mondal, E. Doni, V. Ironmonger, M. Kranz, T. Tuttle and J. A. Murphy, Chem. Sci., 2014, 5, 476 RSC; (m) D. Ghosh, J.-Y. Lee, C.-Y. Liu, Y.-H. Chiang and H. M. Lee, Adv. Synth. Catal., 2014, 356, 406 CrossRef CAS; (n) Y.-W. Zhu, W.-B. Yi, J.-L. Qian and C. Cai, ChemCatChem, 2014, 6, 733 CrossRef CAS; (o) B. S. Bhakuni, A. Yadav, S. Kumar and S. Kumar, New J. Chem., 2014, 38, 827 RSC; (p) Y. Wu, P. Y. Choy and F. Y. Kwong, Org. Biomol. Chem., 2014, 12, 6820 RSC.
- (a) D. S. Roman, Y. Takahashi and A. B. Charette, Org. Lett., 2011, 13, 3242 CrossRef CAS PubMed; (b) C. L. Sun, Y. F. Gu, W. P. Huang and Z. J. Shi, Chem. Commun., 2011, 47, 9813 RSC; (c) M. Rueping, M. Leiendecker, A. Das, T. Poisson and L. Bui, Chem. Commun., 2011, 47, 10629 RSC; (d) B. S. Bhakuni, A. Kumar, S. J. Balkrishna, J. A. Sheikh, S. Konar and S. Kumar, Org. Lett., 2012, 14, 2838 CrossRef CAS PubMed; (e) S. De, S. Ghosh, S. Bhunia, J. A. Sheikh and A. Bisai, Org. Lett., 2012, 14, 4466 CrossRef CAS PubMed; (f) Y. Wu, S. M. Wong, F. Mao, T. L. Chan and F. Y. Kwong, Org. Lett., 2012, 14, 5306 CrossRef CAS PubMed; (g) K.-S. Masters and S. Bräse, Angew. Chem., Int. Ed., 2013, 52, 866 CrossRef CAS PubMed.
- (a) Y. S. Ng, C. S. Chan and K. S. Chan, Tetrahedron Lett., 2012, 53, 3911 CrossRef CAS PubMed; (b) M. E. Buden, J. F. Guastavino and R. A. Rossi, Org. Lett., 2013, 15, 1174 CrossRef CAS PubMed; (c) X. Zheng, L. Yang, W. Du, A. Ding and H. Guo, Chem.–Asian J., 2014, 9, 439 CrossRef CAS PubMed; (d) T. Kawamoto, A. Sato and I. Ryu, Org. Lett., 2014, 16, 2111 CrossRef CAS PubMed.
- J. Cuthbertson, V. J. Gray and J. D. Wilden, Chem. Commun., 2014, 50, 2575 RSC.
- (a) R. A. Rossi, A. B. Pierini and A. B. Peñéñory, Chem. Rev., 2003, 103, 71 CrossRef CAS PubMed; (b) Y. Cheng, X. Gu and P. Li, Org. Lett., 2013, 15, 2664 CrossRef CAS PubMed.
- We propose that primary alkoxy bases (EtOK) can react with aryl iodides much faster than sterically hindered alkoxy bases (t-BuOK) and produce more amount of benzyne and diradical intermediates, though the amount is still problematic to be detected by GC analysis. And the generated diradical intermediates served as electron donors to initiate the SET process, which was proposed in ref. 6l.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07688e |
| This journal is © The Royal Society of Chemistry 2014 |