Influence of the carbon nanofiller surface curvature on the initiation of crystallization in thermoplastic polymers
Abstract
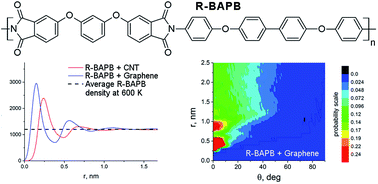
Experimental results have shown that graphitizated carbon nanofibers initiate crystallization in R-BAPB polyimides twice as fast as single-wall carbon nanotubes (CNT) leading to the hypothesis that nanofiller curvature influences polyimide crystallization. Therefore, atomistic molecular-dynamics simulations have been performed for R-BAPB in the presence of a flat graphene sheet and the results were compared with those obtained in the presence of a small-radius CNT. The polyimide chain segments tend to lie parallel to the nanofiller surface and this tendency is stronger and the segments are closer to the graphene surface than to the CNT one. Moreover, the density of the polyimide in the near-surface layer is higher for composites filled with graphene than with CNT. This confirms the assumption that the nanofiller surface curvature is indeed a factor influencing the polymer patterning structure, and that a smaller curvature (i.e. flat surface) provides an enhanced initiation of polymer ordering.

 Please wait while we load your content...
Please wait while we load your content...