Palladium catalyst supported on N-aminoguanidine functionalized magnetic graphene oxide as a robust water-tolerant and versatile nanocatalyst†
Abstract
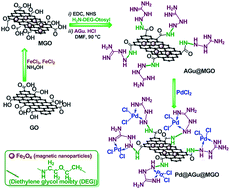
A novel heterogeneous Pd catalyst has been developed by decorating palladium onto the surface of N-aminoguanidine functionalized magnetic graphene oxide nanosheets (denoted as Pd@AGu-MGO), while the diethylene glycol (DEG) group has been applied as an organic spacer. The usefulness of the Pd@AGu-MGO nanocatalyst was investigated in palladium catalyzed organic reactions including Heck/Suzuki couplings of aryl halides and reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). The nanocatalyst showed high efficiency and thermal stability (up to 300 °C). The catalyst recovery test was performed using an external magnet device, and showed that this catalyst can be reused several times without a significant decrease in its performance and catalytic activity. The loading level of Pd in the Pd@AGu-MGO catalyst was assessed as 0.9 mmol g−1 by ICP-AES and elemental analysis. This catalyst is more remarkable from the environmental and economic points of view because of its key properties such as high efficiency and turnover frequency (TOF), mild reaction conditions, utilization of green solvent, simple product work-up, and easy catalyst recovery.

 Please wait while we load your content...
Please wait while we load your content...