Mechanically reinforced composite aerogel blocks by self-growing nanofibers
Abstract
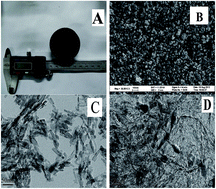
Here we report the preparation of mechanically reinforced composite aerogels using self-growing nanofibers. This approach was used to grow ZrOx nanofibers with a chemical imbalance in the SiO2 precursor solution and establish the property–structure relationship between the mechanical properties and self-growing nanofibers of ZrOx/SiO2 aerogels. As-synthesized ZrOx/SiO2 aerogels show a high compressive strength (9.68 MPa) and high BET surface area (827.22 m2 g−1). These novel ZrOx/SiO2 aerogels could be used as thermal isolates.

 Please wait while we load your content...
Please wait while we load your content...