Rapid and ratiometric sensor for CAN (Ce4+) through metal assisted oxidation reaction-altered through bond energy transfer (TBET): development of low cost devices (TLC plate sticks)†
Shyamaprosad Goswami*,
Sima Paul and
Abhishek Manna
Indian Institute of Engineering Science and Technology, Shibpur (Formerly Bengal Engineering and Science University, Shibpur), Howrah 711103, India. E-mail: spgoswamical@yahoo.com
First published on 5th September 2014
Abstract
The metal catalyzed oxidation reaction in a simple rhodamine–carbazole dyad (a carbazole nucleus flanked by two rhodamine moieties) was carried out in the presence of CAN. This sensor displays a very fast response (<1 min) toward CAN at room temperature with a ‘naked-eye’ change. This TBET based sensor is also highly selective and sensitive towards CAN. The chemosensor showed excellent performance in the “dip-stick” method.
Introduction
Nowadays, much attention has been paid to the development of new fluorescent chemosensors for alkali, alkaline earth and transition metal ions because of their potential applications in the fields of clinical biochemistry, analytical and environmental chemistry.1 Up to now, a significant number of fluorescent sensors for the determination of different oxidizing agents have been documented in literature because of their involvement in biological, therapeutic, imaging processes and catalytic reactions.2 CAN has great antibacterial properties but excess amount of CAN is harmful to biological system.3 Cerium ion has same ionic radius to that of calcium ion and thus it has a higher affinity for Ca2+ binding sites present in biological molecules. Moreover due to its higher charged density it behaves as a hard acid and prefer to the N and O binding sites. Among lanthanide reagents, cerium(IV) ammonium nitrate (CAN) is the most useful one electron oxidant and has been utilized extensively for a broad variety of oxidative transformations in organic chemistry.4 Besides this, CAN is able to catalyze various organic transformations not only based on the Lewis acidic property, but also with electron transfer capability. CAN specially promotes an irreversible oxidative cyclization of the originally N-acylhydrazones into 1,3,4-oxadiazoles in mild conditions Scheme 1.5Recently, much efforts have been focused on the development of rhodamine based probes6 because of the excellent photo physical properties of rhodamine such as high quantum yield, photo stability, absorption and emission in the visible region.7 Ratiometric sensors are exceptionally useful compared to conventional ‘On–Off’ sensors, as the former allow the measurement of emission intensities at two different wavelengths, and thereby, allows the correction for environmental effects. Generally, ratiometric probes can be designed to function following mechanisms: intramolecular charge transfer (ICT),8 fluorescence resonance energy transfer (FRET)9 and two-fluoroionophore sensors.10 Among them, only a FRET-based sensor could provide moderate resolution of the two emission bands. Unfortunately, the efficiency of FRET is primarily controlled by two parameters:11 (1) the limitation in the distance between the energy donor and acceptor fluorophores (e.g., the difference for the classical fluorescein–rhodamine dye pair is fixed at 65 nm) and (2) the spectral overlap between the emission spectra of the energy donor and the absorption spectrum of the energy acceptor. By contrast, in through bond energy transfer (TBET)12 systems, donor and acceptor functional groups that allow attachment of conjugate linkers (for example phenyl, ethynylphenyl and 1,3,4-oxadiazole derivatives) but steric effects prevent them from becoming flat and conjugated. Moreover, TBET is theoretically not subjected to the requirement of spectral overlap between the donor emission and acceptor absorption and is expected to have large Stokes shifts and emission shifts. These spectral benefits are very important for the use of fluorescent dyes in chemistry, biology, medicine, and material science Scheme 2.
Recently Huang et al. reported a highly selective probe for CAN.13 To the best of our knowledge, no TBET-based ratiometric fluorescent probes having rhodamine and carbazole have been reported for CAN. We have reported here the design and synthesis of a new rhodamine–carbazole (RCH) TBET cassette, as a ratiometric probe for CAN. The attachment of a carbazole with rhodamine i.e. dual-switch design for the proposed sensing platform shows two well-separated emission bands for ratiometric CAN sensing. In this case, rhodamine B was chosen as an acceptor because its fluorescence emission is located at long wavelength region and its spirolactam derivatives have been reported to show metal ion-triggered “turn-on” fluorescence signal.14 Carbazole shows an emission at short wavelength area far away from that of rhodamine, which will afford a high resolution for double-channel bio-image. Thus, carbazole was chosen as donor moieties in this case.
The probe RCH was synthesized on the basis of the route shown in Scheme 3. Carbazole dialdehyde and rhodamine hydrazine are synthesized according to reported procedure.15 The structure of the receptor was confirmed by 1H NMR, 13C NMR and HRMS mass spectra.
Experimental section
General
The chemicals and solvents were purchased from Sigma-Aldrich Chemicals Private Limited and were used without further purification. 1H-NMR and 13C-NMR spectra were recorded on Brucker 500 MHz instruments respectively. For NMR spectra, d6-DMSO was used as solvent with TMS as an internal standard. Chemical shifts are expressed in δ units and.1H–1H coupling in Hz. UV-vis titration experiments were performed on a JASCO UV-V530 spectrophotometer and fluorescence experiment was done using PerkinElmer LS 55 fluorescence spectrophotometer with a fluorescence cell of 10 mm path. IR spectra were recorded on a JASCO FT/IR-460 plus spectrometer, using KBr discs.
Method for the preparation of receptor
Synthesis of receptor (RCH)
In a 25 mL flask, rhodamine hydrazine (1 g, 2.19 mmol) and carbazole dialdehyde (365 g, 1.09 mmol) were suspended in 20 mL ethanol. The mixture was refluxed for 12 h with stirring, during which time a light pink precipitate formed. The precipitate was separated by filtration and washed with 3 × 10 mL ethanol. The crude product was then chromatographed on silica gel using CH3Cl–CH3OH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) as the eluent, to afford 887 mg (65%) RCHH as a light pink solid.
5, v/v) as the eluent, to afford 887 mg (65%) RCHH as a light pink solid.
Synthesis of the adduct of CAN and RCH
RCH is mixed with two equivalents of ceric ammonium nitrate (CAN) in acetonitrile at room temperature to give a colorless solution. On removing the solvent, a solid product was obtained which was used for 1H-NMR and MASS spectroscopy.Determination of fluorescence quantum yield
Here, the quantum yield φ was measured by using the following equation.| φx = φs(Fx/Fs)(As/Ax)(nx2/ns2) |
General method of UV-vis and fluorescence titrations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (7
H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The solution of the guest cation was prepared (2 × 10−4 M L−1) in CH3CN
3, v/v). The solution of the guest cation was prepared (2 × 10−4 M L−1) in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (7
H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) at pH 7.4 by using 10 mM HEPES buffer. The original volume of the receptor solution is 2 mL. Solutions of the sensor of various concentrations and increasing concentrations of cations, anions and amine containing compounds were prepared separately. The spectra of these solutions were recorded by means of UV-vis and In fluorescence methods.
3, v/v) at pH 7.4 by using 10 mM HEPES buffer. The original volume of the receptor solution is 2 mL. Solutions of the sensor of various concentrations and increasing concentrations of cations, anions and amine containing compounds were prepared separately. The spectra of these solutions were recorded by means of UV-vis and In fluorescence methods.Results and discussion
UV-vis and fluorescence study
The UV-vis spectra of RCH were measured in CH3CN/water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, 10 mM HEPES, pH 7.4) (Fig. 1a). A new absorption peak at 558 nm appeared upon addition of CAN, together with a remarkable color change from colorless to orange. Such absorption change in the UV-vis region may be ascribed due to the formation of 1,3,4-oxadiazole ring in presence of the oxidizing agent i.e. CAN and the spirolactam ring of the rhodamine moiety was opened. The absorption intensity at 558 nm gradually increased with the increase of the CAN concentration. Other common oxidizing agents (Co2+, Hg2+, Fe3+, I−, IBX, NO3−, NO2−, O2−, OH˙, OCl−, PO43−, SO42−, SO32−, CH3CO3H), however, had no effect on the absorption of probe RCH (Fig. 2). In fact, in presence of 2 equiv. of CAN, a 71 fold enhancement was observed with respect to the CAN free solution (Fig. 1b).
3, v/v, 10 mM HEPES, pH 7.4) (Fig. 1a). A new absorption peak at 558 nm appeared upon addition of CAN, together with a remarkable color change from colorless to orange. Such absorption change in the UV-vis region may be ascribed due to the formation of 1,3,4-oxadiazole ring in presence of the oxidizing agent i.e. CAN and the spirolactam ring of the rhodamine moiety was opened. The absorption intensity at 558 nm gradually increased with the increase of the CAN concentration. Other common oxidizing agents (Co2+, Hg2+, Fe3+, I−, IBX, NO3−, NO2−, O2−, OH˙, OCl−, PO43−, SO42−, SO32−, CH3CO3H), however, had no effect on the absorption of probe RCH (Fig. 2). In fact, in presence of 2 equiv. of CAN, a 71 fold enhancement was observed with respect to the CAN free solution (Fig. 1b).
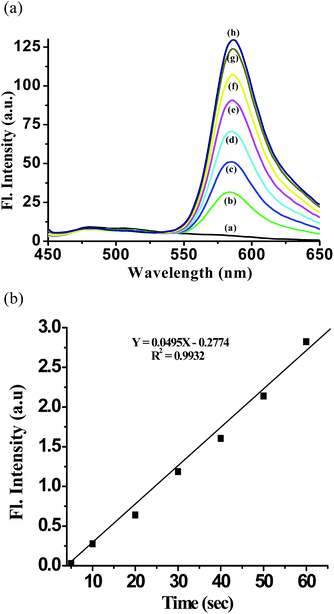
Importantly, when RCH was excited by 450 nm, the intensity of the fluorescence peak at 483 nm gradually decreased and that of a new fluorescence band centered at 585 nm gradually increased, with a well-defined isoemission point at 530 nm (Fig. 3a).
As a result, an obvious change in fluorescence color from blue to red was observed (Fig. 3a inset). This indicated that the ring-opened reaction of the rhodamine B spirolactam and the subsequent TBET process of RCH are triggered by CAN ions. In presence of CAN new 1,3,4-oxadiazole ring was formed in this probe which acts as a conjugate linker during transfer of energy. The mode of energy transfer in receptor RCH is a very fast mechanism operating through bonds, i.e., via this conjugated linker which allows energy transfer from donor to acceptor through bonds. Since the emissions of carbazole and rhodamine B were located at 483 and 585 nm, respectively (Fig. 3a), a wavelength difference of 102 nm is calculated for them, which is larger than that of the classical fluorescein–rhodamine FRET dye pair (65 nm). Moreover, under the present conditions the reaction of the RCH with CAN was rather fast and it reached equilibrium within 1 min after the addition of CAN, with a rate constant of 11.39 × 10−2 s−1, which strongly supports the high reactivity of the probe (Fig. 4).
The energy-transfer efficiencies were calculated based on the equation:16
| ηEET = 1 − ΦF-dyad/ΦF-donor | (1) |
Here, ηEET denotes the efficiency of energy transfer, ΦF-dyad is the fluorescence quantum yield of the dyad (the donor moiety in the TBET system), and ΦF-donor is the fluorescence quantum yield of the donor in the absence of the acceptor. Since, in compound RCH, the carbazole moiety is connected to the rhodamine with a spirolactam form, which shows neither absorption nor emission due to its conjugated system being broken, the ΦF for carbazole moiety in RCH can be ΦF-donor. It was estimated to be 0.017, whereas the ΦF of the carbazole moiety in RCHProduct (ΦF-dyad) was greatly reduced to 0.0015, with quinine sulfate (ΦF = 0.546 in 0.1 M H2SO4) as a standard.15 A ηEET value of 98% was obtained for RCHProduct. This result indicates that a high efficiency of energy transfer of the RCH-based TBET system triggered by CAN. However, the energy transfer is not 100% because some of the flourescence leaks from the carbazole donor rather being transferred to the acceptor. Now, we become interested that the fluorescence response of the sensing system is through a TBET mechanism or not. In this purpose the fluorescence study of probe RCH is carried out in presence of an equimolar mixture of carbazole donor and rhodamine acceptor (ring opened form of rhodamine B) and found that no visible quenching of carbazole moiety and no enhancement in the fluorescence emission of the rhodamine acceptor were observed when the mixture was excited at 450 nm. This result indicates that there is no intermolecular energy transfer between the carbazole donor and rhodamine acceptor in the mixture. Thus, the energy transfer was carried out through TBET mechanism (Scheme 4).
In the presence of CAN, the ratio of emission intensities for rhodamine and to carbazole at 585 and 483 nm (F585/F483) varied from 0.1531 to 14.52, corresponding to a largest signal-to-background ratio (SBR) of 95, which also indicated a high energy transfer efficiency of the TBET system. In contrast, other common metal ions did not show any distinct response (Fig. 5a). Furthermore, the competition experiments revealed that the CAN induced ratiometric fluorescence response was unaffected in the presence of the oxidizing agents mentioned above. Even the presence of I− and CH3CO3H only induced little interference with CAN induced ratiometric fluorescence response (Fig. 5b). All these results indicate that RCH is able to sense CAN in a ratiometric manner in aqueous media with high selectivity. Since, our probe is a reaction based probe, which should theoretically show a higher selectivity toward CAN than that of binding-based probes (Fig. 6).
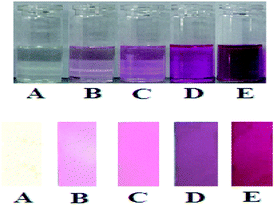 | ||
| Fig. 6 Photograph of RCH towards various concentration of CAN (×10−5 mol) (A) 0, (B) 2, (C) 20, (D) 100, (E) 200 in solution and in TLC plate. | ||
The detection limit was found to be 0.685 μM based on K × Sb1/S, where Sb1 is the standard deviation of blank measurements and S is the slope of the calibration curve. The effects of pH on the fluorescence characteristics of this probe were also investigated (Fig. S5: ESI†). From this curve we see the pKa value is 6 and in a range of pH from 7.0 to 12.0, acidity does not affect the fluorescent intensity of RCH in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (7
H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). So, all the detection carried out using HEPES solution.
3, v/v). So, all the detection carried out using HEPES solution.
Spectral study
To confirm the ratiometric response of the RCH towards CAN, the reaction of the RCH with 2 equiv. CAN was carried out in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). After stirring at ambient temperature for 4 h, the major product was purified by chromatography on a silica gel column and was subjected to 1H NMR, 13C NMR and HRMS mass analysis (ESI†). In 1H NMR spectroscopy interestingly, the sharp peak at 8.943 ppm (for the protons in CH
3, v/v). After stirring at ambient temperature for 4 h, the major product was purified by chromatography on a silica gel column and was subjected to 1H NMR, 13C NMR and HRMS mass analysis (ESI†). In 1H NMR spectroscopy interestingly, the sharp peak at 8.943 ppm (for the protons in CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) completely disappeared and a new proton appeared at 3.610 ppm (for the proton in rhodamine) due to formation of 1,3,4-oxadiazole derivatives.
N) completely disappeared and a new proton appeared at 3.610 ppm (for the proton in rhodamine) due to formation of 1,3,4-oxadiazole derivatives.
Practical application
The sensors for CAN detection can only be performed in solution, which limits their application under special circumstances, such as on-site detection in situ. To demonstrate the practical application of our sensor, we prepared TLC plates of the RCH to determine the suitability of a “dip-stick” method for the detection of CAN. We found that appearance of the red color was observed with increasing concentration of CAN at concentration as low as 20 μM of CAN (Fig. 6) Development of such dipsticks is useful as instant qualitative information is obtained without resorting to instrumental analysis.Conclusions
We have reported a novel easily available turn-on fluorescent chemosensor based on a rhodamine–carbazole conjugate. RCH is a through bond energy transfer based probe for CAN that exhibits excellent selectivity over other oxidizing agents. Moreover, it acts as new chromogenic and fluorescent chemosensor suitable for ratiometric sensing of CAN. This probe rapidly detects CAN at physiological pH of 7.4 in aqueous medium. Here the oxidation reaction was fast at room temperature and irreversible. Dipsticks were also prepared for convenient detection.Acknowledgements
Authors thank the DST and CSIR (Govt. of India) for financial supports. S.P. and A.M. acknowledge the UGC and CSIR respectively for providing fellowships.Notes and references
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS PubMed; W. S. Han, H. Y. Lee, S. H. Jung, S. J. Lee and J. H. Jung, Chem. Soc. Rev., 2009, 38, 1904 RSC; M. Zhu, M. J. Yuan, X. F. Liu, J. L. Xu, J. Lv, C. S. Huang, H. B. Liu, Y. L. Li, S. Wang and D. B. Zhu, Org. Lett., 2008, 10, 1481–1484 CrossRef PubMed.
- P. Das, A. Ghosh and A. Das, Inorg. Chem., 2010, 49, 6909 CrossRef CAS PubMed; C. E. Lisowski and J. E. Hutchison, Anal. Chem., 2009, 81, 10246 CrossRef PubMed; H. Zhang, J. Feng, W. F. Zhu, C. Q. Liu, S. Q. Xu and P. P. Shao, Biol. Trace Elem. Res., 2000, 73, 1 CrossRef; M. Salas, B. Tuchweber, K. Kovacs and B. D. Garg, Effect of cerium on the rat liver, Beitr. Pathol., 1976, 157, 23 CrossRef; W. S. Xia, R. H. Schmehl and C. J. Li, Tetrahedron, 2000, 56, 7045 CrossRef; V. M. Mukkala, M. Helenius, I. Hemmila, J. Kankare and H. Takalo, Helv. Chim. Acta, 1992, 76, 1361 CrossRef PubMed; M. Mwiatkowski, M. Samiotaki, U. Lamminmaki, V. M. Mukkala and U. Landegren, Nucleic Acids Res., 1994, 22, 2604 CrossRef PubMed; M. H. V. Werts, R. H. Woundenberg, P. G. Emmerink, R. van Gassel, J. W. Hofstraat and J. W. Verhoeven, Angew. Chem., Int. Ed., 2000, 39, 4542 CrossRef; S. F. Kirsch and C. Liebert, Eur. J. Org. Chem., 2007, 3711 CrossRef PubMed.
- J. Z. Ni, Bioinorganic chemistry of rare earth elements, 2001, vol. 5 CrossRef CAS; L. H. Nie, H. M. Ma, M. Sun, X. H. Li, M. H. Su and S. C. Liang, Talanta, 2003, 59, 959 CrossRef CAS.
- V. Nair, T. D. Suja and M. Kishor, Tetrahedron Lett., 2005, 46, 3217 CrossRef CAS PubMed; C. Xi, Y. Jing and X. Yang, Tetrahedron Lett., 2005, 46, 3909 CrossRef PubMed; J. R. Hwu and K. Y. King, Curr. Sci., 2001, 8, 1043 Search PubMed; T. L. Ho, Synthesis, 1973, 354 Search PubMed; T. L. Ho, Organic Synthesis by Oxidation with Metal Compounds, Plenum, New York, 1986 Search PubMed; T. Imamoto, Lanthanide Reagents in Organic synthesis, Academic, Londan, 1994, p. 119 Search PubMed.
- M. Dabiri, P. Salehi, M. Baghbanzadeha and M. Bahramnejada, Tetrahedron Lett., 2006, 47, 6983 CrossRef CAS PubMed.
- X. C. Fu, J. Wu, C. G. Xie, Y. Zhong and J. H. Liu, Anal. Methods, 2013, 5, 2615 RSC; B. Bag and B. Biswal, Org. Biomol. Chem., 2012, 10, 2733 Search PubMed; X. Zhang, X. J. Huang and Z. J. Zhu, RSC Adv., 2013, 3, 24891 RSC; A. Banerjee, A. Sahana, S. Lohar, I. Hauli, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Chem. Commun., 2013, 49, 2527–2529 RSC; S. Goswami, S. Paul and A. Manna, RSC Adv., 2013, 3, 10639 RSC; Y. J. Gong, X. B. Zhang, Z. Chen, Y. Yuan, Z. Jin, L. Mei, J. Zhang, W. Tan, G. L. Shen and R. Q. Yu, Analyst, 2012, 137, 932 RSC; M. J. Culzoni, A. M. d. l. Peña, A. Machuca, H. C. Goicoechea and R. Babiano, Anal. Methods, 2013, 5, 30 RSC; V. Luxami, M. Verma, R. Rani, K. Paul and S. Kumar, Org. Biomol. Chem., 2012, 10, 8076 Search PubMed; N. R. Chereddy, K. Saranraj, A. K. Barui, C. R. Patra, V. J. Rao and S. T. Su, RSC Adv., 2014, 4, 24324 RSC; G. Sivaraman and D. Chellappa, J. Mater. Chem. B, 2013, 1, 5768 RSC; G. Sivaraman, T. Anand and D. Chellappa, Analyst, 2012, 137, 5881 RSC; G. Sivaraman and D. Chellappa, J. Mater. Chem. B, 2013, 1, 5768 RSC; G. Sivaraman, V. Sathiyaraja and D. Chellappa, J. Lumin., 2014, 145, 480 CrossRef CAS PubMed; G. Sivaraman, B. Vidya and D. Chellappa, RSC Adv., 2014, 4, 30828 RSC; S. Goswami, A. Manna and S. Paul, RSC Adv., 2014, 4, 21984 RSC; S. Goswami, A. Manna, A. K. Maity, S. Paul, A. K. Das, M. K. Das, P. Saha, C. K. Quah and H. K. Fun, Dalton Trans., 2013, 42, 12844 RSC; S. Goswami, A. K. Das, A. Manna, A. K. Maity, P. Saha, C. K. Quah, H. K. Fun and H. A. Abdel-Aziz, Anal. Chem., 2014, 86, 6315 CrossRef PubMed.
- R. W. Ramette and E. B. Sandell, J. Am. Chem. Soc., 1956, 78, 4872 CrossRef CAS.
- E. A. Jaris-Erijman and T. M. Jovin, Nat. Biotechnol., 2003, 21, 1387–1395 CrossRef CAS PubMed; E. A. Jaris-Erijman and T. M. Jovin, Curr. Opin. Chem. Biol., 2006, 10, 409–416 CrossRef PubMed; C. Lu, Z. Xu, J. Cui, R. Zhang and X. Qian, J. Org. Chem., 2007, 72, 3554 CrossRef PubMed; Z. Xu, K. H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601 CrossRef PubMed; X. Cheng, Q. Li, J. Qin and Z. Li, ACS Appl. Mater. Interfaces, 2010, 2, 1066 Search PubMed; B. C. Zhu, C. C. Gao, Y. Z. Zhao, C. Y. Liu, Y. M. Li, Q. Wei, Z. M. Ma, B. Du and X. L. Zhang, Chem. Commun., 2011, 47, 8656 RSC; G. Sivaraman, T. Anand and D. Chellappa, RSC Adv., 2013, 3, 17029 RSC.
- B. Liu, F. Zeng, G. Wu and S. Wu, Analyst, 2012, 137, 3717 RSC; W. Xuan, Y. Cao, J. Zhou and W. Wang, Chem. Commun., 2013, 49, 10474 RSC; B. Liu, F. Zeng, Y. Liu and S. Wu, Analyst, 2012, 137, 1698 RSC; Y. Wang, D. Gao, P. Zhang, P. Gong, C. Chen, G. Gao and L. Cai, Chem. Commun., 2014, 50, 811 RSC; J. Shao, H. Sun, H. Guo, S. Ji, J. Zhao, W. Wu, X. Yuan, C. Zhang and T. D. James, Chem. Sci., 2012, 3, 1049 RSC; N. R. Chereddy, S. Thennarasu and A. B. Mandal, Analyst, 2013, 138, 1334 RSC; Y. Chen, C. Zhu, J. Cen, J. Li, W. He, Y. Jiao and Z. Guo, Chem. Commun., 2013, 49, 7632 RSC; A. Sahana, A. Banerjee, S. Lohar, A. Banik, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Dalton Trans., 2013, 42, 13311 RSC; S. Lohar, A. Banerjee, A. Sahana, A. Banik, S. K. Mukhopadhyay and D. Das, Anal. Methods, 2013, 5, 442 RSC; B. Chen, P. Wang, Q. Jina and X. Tang, Org. Biomol. Chem., 2014, 12, 5629 Search PubMed; S. Goswami, A. Manna, S. Paul, A. K. Maity, P. Saha, C. K. Quah and H. K. Fun, RSC Adv., 2014, 4, 34572 RSC.
- C. C. Woodroofe and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 11458 CrossRef CAS PubMed; C. Y. Li, X. B. Zhang, L. Qiao, Y. Zhao, C. M. He, S. Y. Huan, L. M. Lu, L. X. Jian, G. L. Shen and R. Q. Yu, Anal. Chem., 2009, 81, 9993 CrossRef PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer, Plenum, New York, 2nd edn, 1999 Search PubMed.
- J. Han, J. Jose, E. Mei and K. Burgess, Angew. Chem., Int. Ed., 2007, 46, 1684 CrossRef CAS PubMed; A. Loudet, R. Bandichhor, L. Wu and K. Burgess, Tetrahedron, 2008, 64, 3462 CrossRef PubMed; Y. Ueno, J. Jose, A. Loudet, C. Pérez-Bolívar, P. Anzenbacher Jr and K. Burgess, J. Am. Chem. Soc., 2011, 133, 51 CrossRef PubMed; W. Lin, L. Yuan, Z. Cao, Y. Feng and J. Song, Angew. Chem., Int. Ed., 2010, 49, 375 CrossRef PubMed; G.-S. Jiao, A. Loudet, H. B. Lee, S. Kalinin, L. B.-A. Johansson and K. Burgess, Tetrahedron, 2003, 59, 3109 CrossRef; D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef PubMed; J. M. Tour, Chem. Rev., 1996, 96, 537 CrossRef PubMed; D. Holten, D. Bocian and J. S. Lindsey, Acc. Chem. Res., 2002, 35, 57 CrossRef PubMed; M. Kumar, N. kumar, V. Bhalla, H. Singh, P. R. Sharma and T. Kaur, Org. Lett., 2011, 13, 1422 CrossRef PubMed; Y. J. Gong, X. B. Zhang, C. C. Zhang, A. L. Luo, T. Fu, W. Tan, G. L. Shen and R. Q. Yu, Anal. Chem., 2012, 84, 10777 CrossRef PubMed.
- L. Huang, Y. Liu, C. Maa, P. Xi, W. Kou and Z. Zeng, Dyes Pigm., 2013, 96, 770 CrossRef CAS PubMed.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC; M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC; X. Q. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed.
- S. Goswami, S. Paul and A. Manna, Dalton Trans., 2013, 42, 10682 RSC; J. F. Zhang, Y. Zhou, J. Yoon, Y. Kim, S. J. Kim and J. S. Kim, Org. Lett., 2010, 12, 3852 CrossRef CAS PubMed.
- G. A. Crosby and J. N. Demas, J. Phys. Chem., 1971, 75, 991 CrossRef CAS; A. Ajayaghosh, P. Carol and S. Sreejith, J. Am. Chem. Soc., 2005, 127, 14962 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07396g |
| This journal is © The Royal Society of Chemistry 2014 |