Pyrite transformation and sulfur dioxide release during calcination of coal gangue†
Abstract
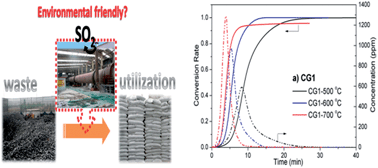
Calcination is a typical process associated with the utilization of coal gangue. A concern associated with this procedure is the emission of sulfur dioxide (SO2). In this work, the behavior of SO2 release during coal gangue calcination under an air atmosphere were systematically investigated and compared to the characteristics of SO2 evolution from pure pyrite calcination. Results show that although sulfur in coal gangue mainly exists in the form of pyrite, it represents different transformation behaviors from that in pure mineral pyrite. At 500 °C, the release rate of SO2 is significantly higher in coal gangue than in mineral pyrite due to the fact that coal gangue combustion can occur at low temperatures, which favors the SO2 release, while at 600 and 700 °C they become almost the same. The shrinking core model cannot describe the SO2 emission profiles in coal gangue and, instead, a hybrid 3D diffusion, i.e. the Jander model, is successfully developed in this study.

 Please wait while we load your content...
Please wait while we load your content...