The reactivity of o-amidophenolate indium(III) complexes towards different oxidants†
Alexandr V. Piskunov*ab,
Irina N. Meshcheryakovaa,
Irina V. Ershovaa,
Artyem S. Bogomyakovc,
Anton V. Cherkasova and
Georgy K. Fukinab
aG.A. Razuvaev Institute of Organometallic Chemistry Russian Academy of Sciences, Tropinina Street 49, 603950 Nizhniy Novgorod, GSP-445, Russian Federation. E-mail: pial@iomc.ras.ru; Fax: +7 831-462-74-97
bN.I. Lobachevsky Nizhny Novgorod State University, 23 Gagarin Avenue, 603950 Nizhny Novgorod, Russian Federation
cInternational Tomography Center, Siberian Branch, Russian Academy of Sciences, Institutskaya Street 3a, 630090 Novosibirsk, Russian Federation
First published on 28th August 2014
Abstract
The reactivity of o-amidophenolate indium(III) complexes towards different oxidants was investigated. The oxidation reactions were found to proceed through the stage of paramagnetic o-iminobenzosemiquinonato indium(III) derivative formation. The monoradical intermediates undergo symmetrization. The final products of the oxidation processes are corresponding biradical o-iminobenzosemiquinonato indium(III) complexes. In order to understand the reasons for the symmetrization processes steric factors (G-parameters) were evaluated for all intermediates and final products by a method based on the ligand solid angle approach.
Introduction
It is well known that the chemical properties of organometallic and coordination compounds are determined by the nature of metal center and the peculiarities of the ligand molecular and electronic structure. The variation of metal and ligand nature allows us to modify finely the reactivity of coordination compounds and create the most effective reagents. The participation of such type ligands in redox processes enables the realization of various unique transformations of main group element compounds. The abilities of such complexes to the reversible binding of small molecules (dioxygen,1 nitrogen monoxide2) and the activation of triple C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds of terminal alkynes3 or C–Hal bonds of alkyl halides4 are examples of transition metal chemistry acquisition realized by the main group metal compounds. It allows to involve the nontransition metal complexes into the various relevant chemical transformations in particular catalytic processes.5
C bonds of terminal alkynes3 or C–Hal bonds of alkyl halides4 are examples of transition metal chemistry acquisition realized by the main group metal compounds. It allows to involve the nontransition metal complexes into the various relevant chemical transformations in particular catalytic processes.5
The diverse chemistry of transition metal complexes based on o-quinone type ligands is under wide investigations in a number of research groups and collected in reviews and recent papers.6 The data concerning such nontransition metal derivatives are quite scarce. The nontransition metal compounds based on dianions of o-quinone type redox active ligands (substituted o-benzoquinones and o-iminobenzoquinones) are known to react readily with different oxidants7 (O-, N-, S-, C-centered radicals, dioxygen, sulfur, halogens, etc.). The paramagnetic radical anion (o-benzosemiquinonate or o-iminobenzosemiquinonate) metal complexes form as a result. These compounds can be detected by EPR spectroscopy. The stability of such paramagnetic species was found to depend on the electronic and sterical effects of substituents bound to metal as well as solvent nature.8 The unstable derivatives undergo symmetrization or reductive elimination of hydrocarbon fragment.7h,8,9 For example, the monoradical indium(III) derivatives based on 3,6-di-tert-butylcatecholate ligand can be prepared by the oxidation of corresponding diolate complexes. The detected paramagnetic species are unstable and undergo the subsequent transformations.9 Recent investigations have shown that the indium(III) complexes can involve all three possible redox forms of the o-iminobenzoquinone ligand depending on the coordination environment of metal.10 The present study is devoted to the investigation of reactivity of o-amidophenolate indium(III) complexes APInI(TMEDA) (1) and [APInEt]2 (2) (where AP is 4,6-di-tert-butyl-N-(2,6-diisopropylphenyl)-o-amidophenonate dianion, TMEDA is N,N,N′N′-tetramethylethylenediamine) towards different oxidants.
Results and discussion
Reactions of o-amidophenolate indium(III) complexes 1 and 2 with different oxidants: characterization of the products
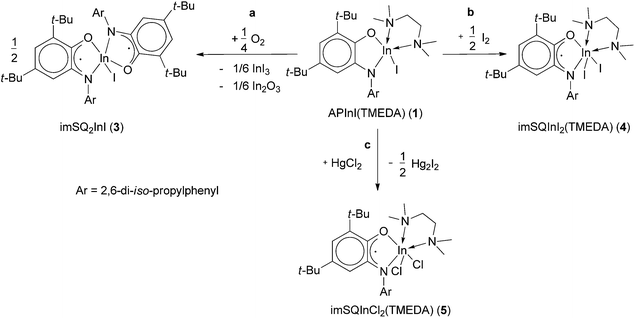
The objects of presented investigation are the recently described o-amidophenolate indium(III) complexes APInI(TMEDA) (1) and [APInEt]2 (2).10b Both of these compounds are tested in the reactions with different oxidative agents (iodine, mercury(II) chloride, tetramethylthiuramedisulphide (TMUDS) and dioxygen) (Schemes 1–3). All interactions are completed in few minutes and accompanied by the change of colour of reaction mixture from orange (for 1) or pale yellow (for 2) to deep green indicating the oxidation of o-amidophenolate ligand into o-iminosemiquinolate one.The o-amidophenolate antimony complexes are known to be reactive towards dioxygen. These reactions occur at mild conditions and result in the formation of corresponding metal containing endoperoxides.1 In contrast to such reactivity, complex 1 reacts with dioxygen and gives paramagnetic derivative as a final product. It was identified as known10a o-iminobenzosemiquinonato indium(III) complex imSQ2InI (3) (where imSQ-4,6-di-tert-butyl-N-(2,6-diisopropylphenyl)-o-iminosemiquinonate ligand) in the accordance with IR-, EPR spectroscopy and elemental analysis (Scheme 1, path a). Such behaviour is very similar to that observed for tin(IV) o-amidophenolates.7h The oxidation of 1 with iodine leads to the formation of another known10b o-iminobenzosemiquinonato indium(III) complex imSQInI2(TMEDA) (4) (Scheme 1, path b). The interaction of 1 with HgCl2 is accompanied with halogen exchange process (Scheme 1, path c). Instead of an expected mixed-halogen derivative, the indium(III) compound imSQInCl2(TMEDA) (5) containing two chlorine atoms bound to the metal centre forms as the result. The presumable driving force for the additional halide exchange is the lower solubility of mercury(I) iodide versus respective chloride.11 The molecular structure of the later was confirmed by X-ray diffraction analysis.
Complex 5 is paramagnetic both in solution and in solid state. The hyperfine structure of X-band EPR spectrum registered in THF (Fig. 1) at 290 K is caused by the interaction of unpaired electron with magnetic nuclei of o-iminobenzosemiquinonato ligand (1H, 99.98%, I = 1/2, μN = 2.7928 and 14N, 99.63%, I = 1, μN = 0.4037(ref. 12)), metal centre (113In, 4.3%, I = 9/2, μN = 5.229 and 115In, 95.7%, I = 9/2, μN = 5.534 (ref. 12)), one of the halogen substituents (35Cl, 75.77%, I = 3/2, μN = 0.8218 and 37Cl, 24.23%, I = 3/2, μN = 0.6841(ref. 12)) and one of the nitrogen atoms of TMEDA molecule. The splitting parameters are the following: ai(1H) = 4.8 G, ai(14N) = 7.4 G, ai(113In) = 12.5 G, ai(115In) = 13.2 G, ai(35Cl) = 0.8 G, ai(37Cl) = 0.7 G, ai(14N) = 1.1 G (gi = 2.0024).
The interaction of APInI2(TMEDA) (1) with TMUDS is also completed during few minutes at moderate heating (40–50 °C) and proceeds through the formation of monoradical o-iminobenzosemiquinonato species imSQInI(SS)(TMEDA) (8) at the first stage (Scheme 2, path a).
The reaction mixture (THF solution at 290 K) is characterized by well resolved EPR spectrum. The hyperfine structure is caused by the interaction of unpaired electron with magnetic nuclei of redox active ligand and metal centre. The splitting parameters are: ai(1H) = 4.9 G, ai(14N) = 6.4 G, ai(115In) = 12.3 G, ai(113In) = 11.6 G (gi = 2.0026). It is reasonable to assume that this paramagnetic product is imSQInI(SS)(TMEDA) (8). We can not unambiguously ascertain the coordination environment of metal centre in this monoradical o-iminobenzosemiquinonato indium(III) derivative 8. The TMEDA molecule and dithiocarbamate ligand can be either mono- or bidentate bound to indium atom. But it is known6f that the value of hyperfine splitting constants on metal magnetic isotopes in the EPR spectra for the related compounds depends on the metal coordination number: it decreases with increasing coordination number. The value of splitting parameter ai(115In) of observed EPR spectrum (12.3 G) for 8 is slightly less than that parameter for complex 5 (ai(115In) = 13.2 G). It indicates that the value of coordination number of the metal centre in imSQInI(SS)(TMEDA) is equal six or more. The intensity of observed isotropic EPR signal decreases until full disappearance during two hours and the biradical complex 6 forms as the result (Scheme 2, path a). Apparently the second product of symmetrization is dithiocarbamate indium(III) diiodide I2InSS which precipitates from the reaction mixture as white powder.
The compound imSQ2InSS (6) containing two o-iminobenzosemiquinonato and one dithiocarbamate ligands is the fine-crystalline dark green solid. The EPR spectrum of 6 in toluene matrix at 150 K is typical for biradical species and the half-field signal (Δms = 2) is observed as well. However both signals (Δms = 1 and Δms = 2) are significantly broadened and the clear determination of zero-splitting parameters is impossible. The broadening can be explained by the presence of additional line splitting on the indium magnetic nuclei.
As mentioned above the intermediate monoradical o-iminobenzoquinonato indium(III) species containing dithiocarbamate ligand imSQInI(SS)(TMEDA) (8) is unstable and undergoes symmetrization. We have made an attempt to obtain monoradical indium(III) derivative containing two dithiocarbamate ligands by the exchange reaction of sodium o-iminosemiquinolate (9) with IInSS2 (Scheme 2, path b). The expected mono-o-iminobenzosemiquinonato indium(III) compound imSQInSS2 (10) forms as an intermediate product on the first stage and was detected using EPR technique. The reaction mixture demonstrates isotropic EPR spectrum in toluene at 290 K (Fig. 2). Its hyperfine structure is caused by the interaction of unpaired electron with magnetic nuclei of the redox active ligand and metal centre. It should be noted that we were able to observe the splitting relating to the proton at carbon atom C(5) of imSQ ligand in the EPR spectrum in the case of imSQInSS2 (10). Usually one cannot observe this kind of hyperfine interaction due to the great line width (2–3 G). The splitting parameters are following: ai(1H) = 4.4 G, ai(1H) = 1.4 G, ai(14N) = 6.9 G, ai(115In) = 12.3 G, ai(113In) = 11.6 G (gi = 2.0027). The biradical compound 6 was also isolated as the final product of this reaction (Scheme 2, path b).
 | ||
| Fig. 2 The X-band EPR spectrum of imSQInSS2 (10) in toluene at 290 K. a – experimental, b – simulated. | ||
The interaction of indium(III) amidophenolate complex [APInEt]2 (2) with iodine, mercury(II) chloride and TMUDS also proceeds through the stage of paramagnetic mono-o-iminobenzosemiquinonato indium(III) species (11–13) formation (Scheme 3, path a, b and d). Each of the derivatives formed was characterized by EPR spectroscopy. The hyperfine structure of EPR signals observed is caused by the interaction of unpaired electron with magnetic nuclei of redox active ligand, metal centre and halogen nuclei (in the case of imSQIn(Et)I (12) and imSQIn(Et)Cl (11)). The splitting parameters are the following: ai(1H) = 4.2 G, ai(14N) = 6.7 G, ai(115In) = 22.9 G, ai(113In) = 21.6 G, ai(127I) = 0.5 G (127I, 100%, I = 5/2, μN = 2.808 (ref. 12)), gi = 2.0021 for imSQIn(Et)I (12) (pentane, 290 K); ai(1H) = 4.6 G, ai(14N) = 6.6 G, ai(115In) = 23.0 G, ai(113In) = 21.7 G, ai(35Cl) = 0.8 G, ai(37Cl) = 0.7 G, gi = 2.0025 for imSQIn(Et)Cl (11)(THF, 290 K); ai(1H) = 4.6 G, ai(14N) = 6.5 G, ai(115In) = 19.1 G, ai(113In) = 18.1 G, gi = 2.0024 for imSQInEt(SS) (13) (THF, 290 K). The EPR spectrum of imSQIn(Et)I (12) in pentane at 290 K is presented on Fig. 3.
 | ||
| Fig. 3 The X-band EPR spectrum of imSQIn(Et)I (12) in pentane at 290K. a – experimental, b – simulated. | ||
It should be noted that the value of the hyperfine splitting constant ai(115In) characteristic for intermediate oxidation products (11–13) of complex 2 exceeds twice the corresponding parameter for analogous derivatives (8, 10) formed as the result of the oxidation of compound 1. It indicates the differences in the coordination centre geometry of intermediate products. The coordination number for derivatives 11–13 is less than that for 8, 10 and equals four (11, 12) or five (13).
The intensity of isotropic EPR signals of 11–13 decreases in time and the spectrum typical for biradical appears in frozen solvent matrix at 150 K. Indeed the final product for any redox reaction (Scheme 3) is compound 7 containing two o-iminobenzosemiquinonato ligands and ethyl group at indium atom. Thus the mono-o-iminobenzosemiquinonato indium(III) derivatives undergo the symmetrization (Scheme 3, path a, and d). The co-products of this symmetrization are EtInCl2, EtInI2 and EtInSS2 (for the path a, b and d respectively). The oxidation of 2 with dioxygen also leads to complex 7 (Scheme 3, path c), however we were unable to register any intermediate products of this reaction.
Complex 7 was characterized by EPR spectrum typical for biradical species (Fig. 4). As in the case of imSQ2InSS (6), the signal shown on Fig. 4 is significantly broadened due to the hyperfine interaction of radical centers with indium magnetic nuclei. Therefore we were unable to determine precisely the zero-splitting parameters.
Magnetic properties of complexes 6 and 7
The magnetochemical investigations for biradical complexes 6 and 7 were performed. The quite different behaviour was observed as a result. Temperature dependences of the effective magnetic moments (μeff) are presented in Fig. 5. The value of μeff at 300 K (2.39 and 2.44 μB for 6 and 7 respectively) is close to spin-only value 2.45 μB typical for system with two non-interacting paramagnetic centres with S = 1/2.At lowering temperature the μeff value of complex 6 decreases insensibly in the range 300–80 K and more suddenly below 80 K and reaches 0.92 μB at 5 K. It indicates the domination of antiferromagnetic exchange interactions between unpaired electrons of o-iminobenzosemiquinonato ligands. Exchange coupled dimer model (H = −2JS1S2) describes experimental data poorly (Fig. 5, dotted line). Indeed in accordance with X-ray diffraction data the molecules of 6 are packed as in chains, and there are short C⋯H contacts (2.7–2.8 Å) between hydrogen atoms of tert-butyl groups of one molecule and carbon atoms of aryl substituent at nitrogen atom of neighbouring molecule. Thus the intramolecular and intermolecular exchange interactions are comparable to each other in fine-crystalline sample of complex 6. So, the uniform chain model (H = −2J∑SiSi+1) was found to be more suitable than the exchange coupled dimer one for description of experimental data. The optimal values of exchange interaction parameters are: J = −8.0 (±0.2) K, g = 2.00 (±0.01).
The value of μeff for 7 increases gradually to 2.58 μB at 17 K and then decreases slightly to 2.41 μB at 5 K. Thus in contrast to complex 6 the compound 7 is characterized by domination of ferromagnetic exchange interactions between spins of two imSQ ligands. Decrease of μeff value near 5 K indicates the presence of weak intermolecular antiferromagnetic exchange interaction in solid sample of 7. Estimation of exchange interaction parameters was carried out using the model of exchange coupled dimer. The optimal values of interaction parameters are: J = 12.9 (±0.3) K, zJ′ = −1.3 (±0.1) K, g = 1.98 (±0.01). It is necessary to note that the similar diradical indium complex 3 demonstrates moderate antiferromagnetic exchange (J = −39.9 (±0.2) K (ref. 9a)) between o-iminosemiquinolate centres. Thus, a change of the ground spin state occurs for the structural analogues 3 and 7 which is caused by the change of the apical substituent at the metal atom. The given fact will be a topic for further research.
Crystal structures of complexes 5–7
The crystal and molecular structures of complexes 5–7 were examined using X-ray diffraction. The selected bond lengths and valence angels are given in Table 1. There are two independent molecules in the unit cell of 5 which differ by the chlorine atoms and TMEDA fragment positions relative to o-iminobenzosemiquinonato ligand. The bond lengths and angles in both molecules are similar and only one of them will be described below. As in 4,10b the indium atom in 5 has a distorted octahedral environment (Fig. 6). The octahedral base is formed by the O(1), N(1), N(3) and Cl(1) atoms while the N(2) and Cl(2) atoms occupy apical positions. The deviation of indium atom from O(1)N(1)N(3)Cl(1) base is 0.15 Å. The value of N(2)–In(1)–Cl(2) angle is 158.24(11)°.| Bond lengths | Valence angles | ||||||
|---|---|---|---|---|---|---|---|
| Complex imSQInCl2(TMEDA) (5) | |||||||
| In(1)–O(1) | 2.163(3) | C(6)–N(1) | 1.337(6) | O(1)–In(1)–N(1) | 75.69(13) | N(3)–In(1)–Cl(2) | 85.50(11) |
| In(1)–N(1) | 2.263(4) | C(1)–C(2) | 1.432(7) | O(1)–In(1)–N(3) | 92.04(14) | Cl(1)–In(1)–Cl(2) | 102.57(6) |
| In(1)–Cl(1) | 2.4063(14) | C(2)–C(3) | 1.370(7) | N(1)–In(1)–N(3) | 167.60(15) | O(1)–In(1)–N(2) | 79.95(13) |
| In(1)–Cl(2) | 2.4649(14) | C(3)–C(4) | 1.421(7) | O(1)–In(1)–Cl(1) | 165.97(10) | N(1)–In(1)–N(2) | 99.96(14) |
| In(1)–N(2) | 2.512(4) | C(4)–C(5) | 1.357(7) | N(1)–In(1)–Cl(1) | 96.65(11) | N(3)–In(1)–N(2) | 75.68(15) |
| In(1)–N(3) | 2.363(4) | C(5)–C(6) | 1.434(7) | N(3)–In(1)–Cl(1) | 94.97(11) | Cl(1)–In(1)–N(2) | 90.03(10) |
| C(1)–O(1) | 1.295(6) | C(1)–C(6) | 1.472(7) | O(1)–In(1)–Cl(2) | 90.07(10) | Cl(2)–In(1)–N(2) | 158.24(11) |
| N(1)–In(1)–Cl(2) | 96.16(11) | ||||||
| Complex imSQ2InSS (6) | |||||||
| In(1)–O(1) | 2.1700(10) | C(2)–C(3) | 1.372(2) | O(2)–In(1)–O(1) | 87.63(4) | N(2)–In(1)–S(1) | 98.79(3) |
| In(1)–N(1) | 2.2561(12) | C(3)–C(4) | 1.437(2) | O(2)–In(1)–N(2) | 74.35(4) | N(1)–In(1)–S(1) | 101.31(3) |
| In(1)–O(2) | 2.1688(10) | C(4)–C(5) | 1.362(2) | O(1)–In(1)–N(2) | 86.02(4) | O(2)–In(1)–S(2) | 103.85(3) |
| In(1)–N(2) | 2.2517(12) | C(5)–C(6) | 1.425(2) | O(2)–In(1)–N(1) | 87.29(4) | O(1)–In(1)–S(2) | 166.12(3) |
| In(1)–S(1) | 2.5606(4) | C(1)–C(6) | 1.460(2) | O(1)–In(1)–N(1) | 74.32(4) | N(2)–In(1)–S(2) | 104.41(3) |
| In(1)–S(2) | 2.5705(4) | C(27)–C(28) | 1.428(2) | N(2)–In(1)–N(1) | 153.68(4) | N(1)–In(1)–S(2) | 98.13(3) |
| C(1)–O(1) | 1.2928(18) | C(28)–C(29) | 1.373(2) | O(2)–In(1)–S(1) | 170.41(3) | S(1)–In(1)–S(2) | 70.972(14) |
| C(6)–N(1) | 1.3390(19) | C(29)–C(30) | 1.425(2) | O(1)–In(1)–S(1) | 98.72(3) | ||
| C(27)–O(2) | 1.2972(17) | C(30)–C(31) | 1.364(2) | ||||
| C(32)–N(2) | 1.3355(18) | C(31)–C(32) | 1.421(2) | ||||
| C(1)–C(2) | 1.434(2) | C(27)–C(32) | 1.466(2) | ||||
| Complex imSQ2InEt (7) | |||||||
| In(1)–O(1) | 2.1836(17) | C(3)–C(4) | 1.430(4) | C(53)–In(1)–N(1) | 122.59(10) | O(1)–In(1)–O(2) | 149.07(7) |
| In(1)–N(1) | 2.177(2) | C(4)–C(5) | 1.362(4) | C(53)–In(1)–O(1) | 106.36(8) | C(53)–In(1)–N(2) | 118.60(10) |
| In(1)–O(2) | 2.1874(17) | C(5)–C(6) | 1.421(4) | N(1)–In(1)–O(1) | 75.16(7) | N(1)–In(1)–N(2) | 118.80(8) |
| In(1)–N(2) | 2.192(2) | C(1)–C(6) | 1.457(3) | C(53)–In(1)–O(2) | 104.52(9) | O(1)–In(1)–N(2) | 88.70(7) |
| In(1)–C(53) | 2.157(3) | C(27)–C(28) | 1.438(4) | N(1)–In(1)–O(2) | 89.73(7) | O(2)–In(1)–N(2) | 74.98(7) |
| C(1)–O(1) | 1.297(3) | C(28)–C(29) | 1.379(4) | ||||
| C(6)–N(1) | 1.345(3) | C(29)–C(30) | 1.442(4) | ||||
| C(27)–O(2) | 1.289(3) | C(30)–C(31) | 1.357(4) | ||||
| C(32)–N(2) | 1.352(3) | C(31)–C(32) | 1.421(4) | ||||
| C(1)–C(2) | 1.434(4) | C(27)–C(32) | 1.456(4) | ||||
| C(2)–C(3) | 1.370(4) | ||||||
 | ||
| Fig. 6 The molecular structure of 5 with 50% thermal probability ellipsoids. The H atoms are omitted for clarity. | ||
The values of In(1)–N(2) (2.512(4) Å) and In(1)–N(3) (2.363(4) Å) distances exceed the sum of covalent radii of these elements (2.17 Å (ref. 13)) but less than the sum of Van der Waals radii (4.2 Å (ref. 13)), thus these bonds have donor–acceptor nature.
It should be noted that the In(1)–N(3) bond is shorter than the In(1)–N(2). The same situation is observed for In–Cl bonds where the In(1)–Cl(1) distance (2.4063(14) Å) is less than the In(1)–Cl(2) (2.4649(14) Å). It is caused by the location of Cl(2) and N(3) atoms in the apical positions. The In(1)–N(2) and In(1)–Cl(2) bonds are orthogonal to the imSQ ligand plane and these Cl(2) and N(3) atoms participate in the hyperfine interaction with unpaired electron that is shown in the hyperfine structure of the EPR spectrum of 5.
In accordance with X-ray diffraction data (Fig. 7) the coordination polyhedron of indium atom in 6 is a distorted octahedron. The O(1), O(2), S(1) and S(2) atoms form the octahedron base while the N(1) and N(2) atoms occupy apical sites. The o-iminoquinolate ligands are located in such a way where the nitrogen atoms are in trans-positions and the N(1)–In(1)–N(2) angle is 153.68(4)°. The C6H2O(1)N(1) and C6H2O(2)N(2) planes form the dihedral angle 53.02°.
 | ||
| Fig. 7 The molecular structure of 6 with 50% thermal probability ellipsoids. The H atoms, methyl groups of tert-butyl and iso-propyl substituents are omitted for clarity. | ||
There are two crystallographically unique molecules in the crystal cell of 7. These molecules differ from each other by relative positions of ethyl groups and two redox active ligands.
The presence of two asymmetric chelate imSQ ligands in five-coordinating complex causes the chirality of metal centre and appearance of several isomers. This situation was previously observed for pentacoordinated indium and tin complexes based on imQ ligand.10a,7h
The crystal cell of 7 contains the solvate molecule of DME with the ratio “complex![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent” as 2
solvent” as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. The coordination polyhedron is a distorted trigonal bipyramid (Fig. 8). The N(1), N(2) and C(53) atoms lie in the bipyramid base and O(1), O(2) atoms occupy apical sites. The O(1)–In(1)–O(2) angle is 149.07(7)°, the indium atom is neatly located in the N(1)N(2)C(53) plane. As in complex 6, the arrangement of redox active ligands results in trans-position of the nitrogen atoms. The dihedral angle between C6H2O(1)N(1) and C6H2O(2)N(2) planes is 44.47°.
1.5. The coordination polyhedron is a distorted trigonal bipyramid (Fig. 8). The N(1), N(2) and C(53) atoms lie in the bipyramid base and O(1), O(2) atoms occupy apical sites. The O(1)–In(1)–O(2) angle is 149.07(7)°, the indium atom is neatly located in the N(1)N(2)C(53) plane. As in complex 6, the arrangement of redox active ligands results in trans-position of the nitrogen atoms. The dihedral angle between C6H2O(1)N(1) and C6H2O(2)N(2) planes is 44.47°.
 | ||
| Fig. 8 The molecular structure of 7 with 50% thermal probability ellipsoids. The H atoms, methyl groups of iso-propyl substituents are omitted for clarity. | ||
The geometry of imSQ ligands in 5–7 is typical for radical anion form of such type redox-active ligand and comparable with those in known o-iminobenzosemiquinonato metal complexes.14 Thus the C(1)–O(1) and C(6)–N(1) bond lengths have intermediate value between values characteristic for corresponding single and double bonds. The In(1)–O(1) and In(1)–N(1) distances are equal or exceed slightly the sums of covalent radii of corresponding elements (2.16 Å for In–O and 2.17 Å for In–N13) and typical for radical anion form of imQ ligand coordination in previously reported indium derivatives.9 The o-quinone alternation in six-membered C(1)–C(6) carbon ring is also observed. It appears in the separation of shorter C(2)–C(3) and C(4)–C(5) (1.357(7)–1.370(7) Å) bonds by longer C(1)–C(2), C(3)–C(4), C(5)–C(6) and C(1)–C(6) (1.421(7)–1.472(7) Å) bonds.
The evaluation of steric hindrances in the metal coordination sphere of indium o-iminosemiquinonate derivatives
Mono-o-iminobenzosemiquinonato indium(III) compounds are unstable and undergo subsequent symmetrization with the formation of biradical products in most cases as it was shown above. Notably, in contrast to indium imQ derivatives described herein, the symmetrization of related mono-o-benzosemiquinonato indium(III) complexes leads mainly to the triradical compound (3,6-SQ)3In (3,6-SQ is radical anion form of 3,6-di-tert-butyl-o-benzoquinone).9The sterical situation in coordination sphere of metal is known to be one of the factors determining the final structure. Therefore we carried out the quantitative estimation of the shielding of the central metal atom based on the ligand solid angle approach (G-parameter15) for intermediate and resulting complexes. Previously, this approach allowed to explain the formation, stability and reactivity of a number of coordination compounds16 including o-iminosemiquinonates.8d The geometric characteristics necessary for calculation of G-parameter were taken from X-ray diffraction data for imSQ2InI (3),10a imSQInI2(TMEDA) (4),10b imSQInCl2(TMEDA) (5), imSQ2InSS (6), imSQ2InEt (7). The geometry of unstable intermediate derivatives was optimized using DFT calculations. Calculations were performed at the B3LYP/3-21G level. Additional calculations of G-parameters for optimized geometries of 3–7 were made in order to evaluate the adequacy of the chosen level of theory. It is seen (Table 2) that the calculated structural parameters are in good agreement with experimental.
| Complex | G, % |
|---|---|
| 3 | 85.7(2) [86.9(2)] |
| 4 | 89.6(2) [91.3(2)] |
| 5 | 88.7(2) [88.9(2)] |
| 6 | 90.2(2) [90.7(2)] |
| 7 | 87.6(2) [87.4(2)] |
| imSQInI(SS) | 74.9(2) |
| 10 | 81.3(2) |
| 11 | 65.1(2) |
| 12 | 66.4(2) |
| 13 | 76.1(2) |
| imSQ2InCl | 85.8(2) |
| imSQInI2 | 65.0(2) |
| imSQInI(Et)(TMEDA) | 91.2(2) |
The oxidation of 1 with O2, I2 and HgCl2 leads to the formation of stable compounds imSQ2InI (3), imSQInI2(TMEDA) (4) and imSQInCl2(TMEDA) (5) respectively. The values of G-parameter for these isolated products are in a fairly narrow range 86–90%.
As mentioned above we were unable to unambiguously determine the composition of mono-o-iminobenzosemiquinonato indium(III) specie formed as the result of oxidation of 1 with TMUDS. The modeling of the geometric parameters for imSQInI(SS)(TMEDA) (8) complex as sum of ligand solid angels has shown that the saturation of the metal coordination sphere by ligands exceeds 100%, i.e. the seven-coordinated compound should not exist in such a form (Scheme 4). Possibly it loses the TMEDA molecule that leads to the coordinatively unsaturated (G = 74.9(2) %) and unstable imSQInI(SS) species. Thus the monoradical indium(III) derivative should undergo symmetrization to form the stable product. Two ways of symmetrization are possible (Scheme 4). The first one leads to complex imSQ2InI (3) and the second one – to imSQ2InSS (6). In the accordance with values of G-parameter, both these compounds can be stable and the choice of symmetrization way is probably determined by the solubility of second products (IInSS2 and I2InSS) (Scheme 4).
The reason of symmetrization of monoradical complex imSQInSS2 (10) (G = 81.3(2) %) is also its coordination unsaturation and 10 transforms into imSQ2InSS (6).
The intermediate mono-o-iminobenzosemiquinonato indium(III) derivatives 11–13 are characterized by low G-parameters that causes their symmetrization too (Scheme 5).
There are two ways of symmetrization for unstable compounds 11–13. The first one leads to imSQ2InEt (7). The second one results in the formation of imSQ2InX (X is Cl or I) in the case of 11 and 12 or imSQ2InSS (6) in the case of 13. All these biradical indium(III) derivatives are stable (G-parameters are in the range 86–90%). The formation of complex 7 as the final product of symmetrization of 11–13 (Scheme 5) can be explained by the lower solubility of co-products EtInX2 vs.Et2InX and EtInSS2 vs.Et2InSS. The precipitation of the monoalkylindium derivatives promotes the formation of 7.
We have analyzed additional model compound imSQInI2. It is known that the reaction of equimolar amounts of imSQNa with InI3 leads to the formation of imSQ2InI (3) as the result of symmetrization of monoradical complex imSQInI2.10a The tetracoordinated compound has G-parameter equal to 65.0(2) % and is unstable.
Conclusions
In summary, in the course of present study it was found that the oxidation of indium o-amidophenolate complexes 1 and 2 proceeds through the formation of monoradical o-iminobenzosemiquinonato metal derivatives 8,10–13 which can be detected in solutions using EPR. In most cases such intermediate indium(III) species undergo the symmetrization due to their coordination unsaturation that leads to the corresponding stable biradical complexes. The executed calculations of the percentage of the metal coordination sphere shielded by all ligands (G-parameter) for series of indium(III) o-iminobenzosemiquinonato complexes described in this report indicates that the optimal values of G-parameter for stable derivatives are in the range of 86–90%.Experimental
Experimental details
All reactants were purchased from Aldrich. Solvents were purified by standard methods.17 The following reactants sodium o-iminobenzosemiquinolate imSQNa,10a InI,18 1, 210b and I2InEt19 were prepared according to the known procedures. All manipulations on complexes were performed in vacuum under conditions in which oxygen and moisture were excluded.The infrared spectra of complexes in the 4000–400 cm−1 range were recorded on a FSM 1201 Fourier-IR spectrometer in nujol. EPR spectra were recorded by using a Bruker EMX spectrometer (working frequency ≈ 9.75 GHz). The gi values were determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) as the reference (gi = 2.0037). EPR spectra were simulated with the WinEPR SimFonia Software (Bruker). The elemental analysis was performed on an Elemental Analyzer Euro EA 3000 instrument.
The magnetic susceptibility of the polycrystalline complexes was measured with a Quantum Design MPMSXL SQUID magnetometer in the temperature range 2–300 K with magnetic field of up to 5 kOe. None of complexes exhibited any field dependence of molar magnetization at low temperatures. Diamagnetic corrections were made using the Pascal constants. The effective magnetic moment was calculated as μeff(T) = [(3k/NAμB2)χT]1/2 ≫ (8χT)1/2.
The oxidation of 1 with dioxygen
The solution of complex 1 (0.35 g, 0.47 mmol) in THF (25 ml) was exposed to the fixed volume of dry dioxygen (50 ml). The reaction mixture was stirred for few minutes during that the color changed from orange to deep green. The THF was replaced with hexane (15 ml). The crystalline product imSQ2InI (3) was isolated from the reaction mixture after storage of the later at −18 °C overnight. The total yield of analytically pure compound is 0.14 g (58%).The oxidation of 1 with iodine
The solution of complex 1 (0.35 g, 0.47 mmol) in THF (25 ml) was added to the solution of I2 (0.0596 g, 0.235 mmol) in the same solvent (3 ml). The reaction mixture turned brown. The THF was removed under reduced pressure. The solid residue was dissolved in diethyl ether (15 ml). The solution was stored at −18 °C overnight. It led to the formation of brown-green crystals of imSQInI2(TMEDA) (4). The total yield of analytically pure compound is 0.27 g (61%).The interaction of 1 with HgCl2
The solution of complex 1 (0.35 g, 0.47 mmol) in THF (25 ml) was added to the solution of HgCl2 (0.1276 g, 0.47 mmol) in the same solvent (5 ml). The reaction mixture was stirred during 15–20 minutes. The solution color gradually changed from orange to deep blue. The THF was replaced by hexane (15 ml). The white-yellow precipitate of Hg2I2 was removed by filtration. The following storage of the solution at −18 °C during few hours led to the formation of crystalline blue-green product imSQInCl2(TMEDA) (5). The total yield of analytically pure compound is 0.21 g (65%).Anal. calc. for C32H53Cl2InN3O: C, 56.40; H, 7.84; Cl, 10.40; In, 16.85%. Found: C, 56.67; H, 7.95; Cl, 10.34; In, 16.49%. IR (Nujol, KBr) cm−1: 1588 (w), 1442 (s), 1426 (s), 1407 (m), 1364 (m), 1351 (m), 1327 (m), 1319 (m), 1306 (w), 1288 (w), 1270 (w), 1252 (m), 1213 (m), 1198 (w), 1181 (w), 1164 (w), 1124 (w), 1120 (w), 1102 (w), 1056 (w), 1046 (w), 1024 (m), 1011 (m), 992 (w), 952 (m), 937 (w), 913 (w), 888 (w), 866 (m), 817 (w), 797 (s), 772 (m), 768 (m), 743 (w), 707 (w), 667 (w), 644 (w), 623 (w), 610 (w), 589 (w), 574 (w), 539 (w), 529 (w), 497 (w), 478 (w).
The oxidation of 1 with TMUDS
The solution of 1 (0.35 g, 0.47 mmol) in THF (25 ml) was added to the solution of TMUDS (0.0565 g, 0.235 mmol) in the same solvent (5 ml). The reaction mixture was stirred during 15–20 minutes at 40–50 °C. The solution color changed from orange to deep green. The THF was removed under reduced pressure. The solid residue was dissolved in hexane (15 ml). The obtained solution was kept during an hour, the formation of white precipitate was observed. The solution was separated from I2InSS by filtration and stored at −18 °C overnight. The deep green crystals of imSQ2InSS (6) were obtained as the result. The total yield of analytically pure compound is 0.16 g (69%).Anal. calc. for C55H80InN3O2S2: C, 66.44; H, 8.11; In, 11.55; S, 6.45%. Found: C, 66.79; H, 8.26; In, 11.40; S, 6.21%. IR (Nujol, KBr) cm−1: 1585 (m), 1510 (m), 1444 (s), 1430 (s), 1412 (m), 1362 (s), 1352 (s), 1330 (s), 1323 (m), 1307 (m), 1267 (w), 1251 (s), 1216 (w), 1198 (m), 1167 (m), 1136 (w), 1113 (w), 1098 (w), 1055 (w), 1042 (w), 1026 (w), 992 (w), 979 (m), 936 (w), 924 (w), 910 (w), 884 (w), 866 (m), 822 (w), 803 (m), 797 (m), 776 (w), 772 (w), 763 (w), 744 (w), 705 (w), 665 (w), 646 (w), 626 (w), 604 (w), 571 (w), 538 (w), 528 (w), 496 (w), 476 (w), 463 (w).
The exchange interaction of imSQNa with IInSS2
Anal. calc. for C6H12IInN2S4: C, 14.95; H, 2.51; I, 26.32; In, 23.81; S, 26.60. Found: C, 15.13; H, 2.75; I, 26.18; In, 23.76; S, 26.41.
The solution of imSQNa (0.4 g, 0.994 mmol) in THF (25 ml) was added to the solution of IInSS2 (0.479 g, 0.994 mmol) in the same solvent (10 ml). The reaction mixture turned deep green. The THF was replaced by hexane (15 ml). The solution was kept during an hour at room temperature and the formation of white precipitate InSS3 (ref. 20) was observed. The precipitate was removed by filtration. The resulted solution was stored at −18 °C overnight that led to the formation of crystalline product imSQ2InSS (6). The total yield of analytically pure compound is 0.28 g (57%).
The oxidation of [APInEt]2 (2) with HgCl2
The solution of 2 (0.35 g, 0.334 mmol) in THF (20 ml) was added to the solution of HgCl2 (0.1814 g, 0.668 mmol) in the same solvent (10 ml). The reaction mixture was stirred during 15–20 minutes at room temperature. The color changed from orange to deep green and the precipitation of Hg2Cl2 was observed. The deposit was removed by filtration. The THF was replaced by hexane (15 ml). The reaction mixture was kept during an hour at ambient temperature and the precipitation of EtInCl2 was observed. The EtInCl2 was removed by filtration. The following storage of the solution at −18 °C led to formation of crystalline product imSQ2InEt·0.75(hexane) (7·0.75(hexane)). The total yield of analytically pure compound is 0.17 g (53%).Anal. calc. for C58.5H89.5InN2O2: C, 72.61; H, 9.32; In, 11.87%. Found: C, 72.86; H, 9.55; In, 11.62. IR (Nujol, KBr) cm−1: 1588 (s), 1467 (s), 1442 (s), 1435 (s), 1360 (s), 1355 (s), 1334 (s), 1320 (m), 1255 (s), 1214 (w), 1198 (m), 1170 (m), 1112 (m), 1102 (m), 1056 (m), 1042 (w), 1026 (m), 1009 (w), 992 (m), 880 (w), 873 (m), 861 (m), 820 (w), 797 (s), 779 (w), 764 (m), 744 (w), 709 (w), 665 (w), 648 (w), 638 (m), 626 (w), 607 (w), 585 (w), 574 (w), 539 (w), 527 (w), 497 (w), 477 (w).
The oxidation of [APInEt]2 (2) with I2
The solution of 2 (0.35 g, 0.334 mmol) in THF (20 ml) was added to the solution of I2 (0.0848 g, 0.334 mmol) in the same solvent (5 ml). The reaction mixture turned deep green. The replacement of THF by hexane (15 ml) led to the formation of EtInI2 deposit. The solution was kept during an hour and separated from EtInI2 by filtration. The result product imSQ2InEt·0.75(hexane) (7·0.75(hexane)) was isolated according to the method described above. The total yield of analytically pure compound is 0.21 g (65%).The oxidation of [APInEt]2 (2) with dioxygen
The solution of 2 (0.35 g, 0.334 mmol) in THF (20 ml) was exposed to dry dioxygen (50 ml). The reaction mixture turned deep green during few minutes. The THF was replaced with hexane (15 ml). The result complex imSQ2InEt·0.75(hexane) (7·0.75(hexane)) was isolated according to the method described above. The total yield of analytically pure compound is 0.20 g (62%).The interaction of [APInEt]2 (2) with TMUDS
The solution of 2 (0.35 g, 0.334 mmol) in THF (20 ml) was added to the solution of TMUDS (0.08 g, 0.334 mmol) in the same solvent (7 ml). The reaction mixture was stirred during 15–20 minutes at 40–50 °C. The solution color changed from pale yellow to deep green. The THF was removed under reduced pressure and the solid residue was dissolved in hexane (15 ml). The reaction mixture was kept during an hour and the formation of white precipitate EtInSS2 was observed. The precipitate was removed by filtration. The resulting complex imSQ2InEt·0.75(hexane) (7·0.75(hexane)) was isolated according to the method described above. The total yield of analytically pure compound is 0.20 g (60%).X-ray crystallographic studies of imSQInCl2(TMEDA) (5), imSQ2InSS (6) and imSQ2InEt·0.75(DME) (7·0.75(DME))
The single crystals suitable for X-ray diffraction analysis were obtained from hexane (for 5 and 6) or DME (for 7·0.75(DME)). The intensity data were collected at 150 K (for 5 and 6) and 100 K (for 7·0.75(DME)) on a Smart Apex diffractometer with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) in the φ–ω scan mode (ω = 0.3°, 10 s on each frame). The intensity data were integrated by SAINT program.21 SADABS22 was used to perform area-detector scaling and absorption corrections. The structures were solved by direct methods and were refined on F2 using all reflections with SHELXTL package.23 All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were placed in calculated positions and refined in the “riding-model”. Selected bond lengths and angles of complexes are given in Table 1. Table 3 summarises the crystal data and some details of the data collection and refinement.| Complex | imSQInCl2(TMEDA) (5) | imSQ2InSS (6) | imSQ2InEt·0.75(DME) (7·0.75(DME)) |
|---|---|---|---|
| Empirical formula | C32H53Cl2InN3O | C55H80InN3O2S2 | C57H86.50InN2O3.50 |
| Formula weight | 681.49 | 994.16 | 970.60 |
| Temperature [K] | 150(2) | 150(2) | 100(2) |
| Wavelength [Å] | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Triclinic | Monoclinic |
| Space group | P2(1)/n | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P2(1)/n |
| Unit cell dimensions, [Å], [°] | a = 11.7294(9), b = 17.9283(13), c = 32.527(2), α = 90, β = 96.017(2), γ = 90 | a = 11.8378(8), b = 13.5406(9), c = 17.8321(11), α = 101.5320(10), β = 92.0740(10), γ = 99.2270(10) | a = 20.5030(6), b = 22.1273(6), c = 25.5483(7), α = 90, β = 106.8230(10), γ = 90 |
| Volume [Å3] | 6802.3(9) | 2757.5(3) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094.6(5) 094.6(5) |
| Z | 8 | 2 | 8 |
| Density (calculated) [g cm−3] | 1.331 | 1.197 | 1.162 |
| Absorption coefficient [mm−1] | 0.880 | 0.544 | 0.468 |
| Crystal size [mm3] | 0.40 × 0.27 × 0.15 | 0.57 × 0.26 × 0.15 | 0.27 × 0.26 × 0.21 |
| Theta range for data collection [°] | 1.79 to 26.00 | 1.74 to 27.00 | 1.84 to 26.00 |
| Reflections collected | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 742 742 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 144 |
| Independent reflections | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 342 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 942 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
| R(int) | 0.0692 | 0.0194 | 0.0768 |
| Completeness to theta max | 99.7 | 99.3 | 99.5 |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 0.8794 and 0.7199 | 0.9229 and 0.7468 | 0.9081 and 0.8840 |
| Refinement method | Full-matrix least-squares on F^2 | Full-matrix least-squares on F^2 | Full-matrix least-squares on F^2 |
| Data/restraints/parameters | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342/0/731 342/0/731 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942/0/590 942/0/590 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728/58/1211 728/58/1211 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0647, wR2 = 0.1331 | R1 = 0.0315, wR2 = 0.0772 | R1 = 0.0537, wR2 = 0.1223 |
| R indices (all data) | R1 = 0.0984, wR2 = 0.1432 | R1 = 0.0385, wR2 = 0.0801 | R1 = 0.0879, wR2 = 0.1344 |
| Goodness-of-fit on F2 | 1.073 | 1.073 | 1.004 |
| Largest diff. peak and hole [e Å−3] | 2.752 and −1.115 | 0.737 and −0.437 | 1.583 and −0.699 |
Density functional theory calculations
DFT calculations were performed with the GAUSSIAN 03 (ref. 24) program package using the B3LYP/3-21G level of theory. The absence of imaginary frequencies after the optimization procedure suggests that the molecular geometries correspond to the energy minima.Acknowledgements
We are grateful to the Russian Scientific Foundation (grant 14-03-01296) for financial support of this work.Notes and references
- (a) G. A. Abakumov, A. I. Poddel'sky, E. V. Grunova, V. K. Cherkasov, G. K. Fukin, Y. A. Kurskii and L. G. Abakumova, Angew. Chem., Int. Ed, 2005, 44, 2767–2771 CrossRef CAS PubMed; (b) V. K. Cherkasov, G. A. Abakumov, E. V. Grunova, A. I. Poddel'sky, G. K. Fukin, E. V. Baranov, Y. A. Kursky and L. G. Abakumova, Chem.–Eur. J., 2006, 12, 3916–3927 CrossRef CAS PubMed; (c) A. I. Poddel'sky, Y. A. Kuskii, A. V. Piskunov, N. N. Somov, V. K. Cherkasov and G. A. Abakumov, Appl. Organomet. Chem., 2011, 25, 180–189 CrossRef PubMed.
- E. V. Ilyakina, A. I. Poddel'sky, V. K. Cherkasov and G. A. Abakumov, Mendeleev Commun., 2012, 22, 208–210 CrossRef CAS PubMed.
- I. L. Fedushkin, A. S. Nikipelov and K. A. Lyssenko, J. Am. Chem. Soc., 2010, 132, 7874–7875 CrossRef CAS PubMed.
- (a) A. V. Piskunov, I. N. Meshcheryakova, G. K. Fukin, A. S. Shavyrin, V. K. Cherkasov and G. A. Abakumov, Dalton Trans., 2013, 42, 10533–10539 RSC; (b) A. V. Piskunov, I. V. Ershova, G. K. Fukin and A. S. Shavyrin, Inorg. Chem. Commun., 2013, 38, 127–130 CrossRef CAS PubMed.
- (a) W. I. Dzik, J. I. van der Vlugt, J. N. H. Reek and B. de Bruin, Angew. Chem., Int. Ed., 2011, 50, 3356–3358 CrossRef CAS PubMed; (b) I. L. Fedushkin, A. S. Nikipelov, A. G. Morozov, A. A. Skatova, A. V. Cherkasov and G. A. Abakumov, Chem.–Eur. J., 2012, 18, 255–266 CrossRef CAS PubMed; (c) P. J. Chirik and K. Wieghardt, Science, 2010, 327, 794–795 CrossRef CAS PubMed; (d) O. R. Luca and R. H. Crabtree, Chem. Soc. Rev., 2013, 42, 1440–1459 RSC.
- (a) C. G. Pierpont, Coord. Chem. Rev., 2001, 219–221, 415–433 CrossRef CAS; (b) C. G. Pierpont, Coord. Chem. Rev., 2001, 216–217, 99–125 CrossRef CAS; (c) A. I. Poddel'sky, V. K. Cherkasov and G. A. Abakumov, Coord. Chem. Rev., 2009, 253, 291–324 CrossRef PubMed; (d) W. Kaim, Inorg. Chem., 2011, 50, 9752–9765 CrossRef CAS PubMed; (e) N. Deibel, D. Schweinfurth, S. Hohloch, J. Fiedler and B. Sarkar, Chem. Commun., 2012, 48, 2388–2390 RSC; (f) E. M. Matson, S. M. Franke, N. H. Anderson, T. D. Cook, P. E. Fanwick and S. C. Bart, Organometallics, 2014, 33, 1964–1971 CrossRef CAS; (g) D. Das, H. Agarwala, A. D. Chowdhury, T. Patra, S. M. Mobin, B. Sarkar, W. Kaim and G. K. Lahiri, Chem.–Eur. J., 2013, 19, 7384–7394 CrossRef CAS PubMed; (h) S. Ghorai and C. Mukherjee, Chem. Commun., 2012, 48, 10180–10182 RSC; (i) S. E. Balaghi, E. Safaei, L. Chiang, E. W. Y. Wong, D. Savard, R. M. Clarke and T. Storr, Dalton Trans., 2013, 42, 6829–6839 RSC; (j) N. Deibel, D. Schweinfurth, S. Hohloch, M. Delor, I. V. Sazanovich, M. Towrie, J. A. Weinstein and B. Sarkar, Inorg. Chem., 2014, 53, 1021–1031 CrossRef CAS PubMed; (k) A. H. Randolph, N. J. Seewald, K. Rickert and S. N. Brown, Inorg. Chem., 2013, 52, 12587–12598 CrossRef CAS PubMed; (l) S. Sproules, R. R. Kapre, N. Roy, T. Weyhermüller and K. Wieghardt, Inorg. Chim. Acta, 2010, 363, 2702–2714 CrossRef CAS PubMed.
- (a) T. A. Annan and D. G. Tuck, Can. J. Chem., 1989, 67, 1807–1814 CrossRef CAS; (b) H. E. Mabrouk and D. G. Tuck, J. Chem. Soc., Dalton Trans., 1988, 2539 RSC; (c) G. M. Barnard, M. A. Brown, H. E. Mabrouk, B. R. McGarvey and D. G. Tuck, Inorg. Chim. Acta, 2003, 349, 142–148 CrossRef CAS; (d) A. V. Lado, A. I. Poddel'sky, A. V. Piskunov, G. K. Fukin, E. V. Baranov, V. N. Ikorskii, V. K. Cherkasov and G. A. Abakumov, Inorg. Chim. Acta, 2005, 358, 4443–4450 CrossRef CAS PubMed; (e) G. A. Abakumov, V. K. Cherkasov, A. V. Piskunov, A. V. Lado, G. K. Fukin and L. G. Abakumova, Russ. Chem. Bull., 2006, 55, 1146–1154 CrossRef CAS; (f) A. V. Lado, A. V. Piskunov, V. K. Cherkasov, G. K. Fukin and G. A. Abakumov, Russ. J. Coord. Chem., 2006, 32, 173–179 CrossRef CAS; (g) A. V. Piskunov, A. V. Lado, E. V. Ilyakina, G. K. Fukin, E. V. Baranov, V. K. Cherkasov and G. A. Abakumov, J. Organomet. Chem., 2008, 693, 128–134 CrossRef CAS PubMed; (h) A. V. Piskunov, I. N. Mescheryakova, G. K. Fukin, E. V. Baranov, M. Hummert, A. S. Shavyrin, V. K. Cherkasov and G. A. Abakumov, Chem.–Eur. J., 2008, 14, 10085–10093 CrossRef CAS PubMed; (i) E. V. Ilyakina, A. I. Poddel'sky, A. V. Piskunov, N. V. Somov, V. K. Cherkasov and G. A. Abakumov, Inorg. Chim. Acta, 2012, 380, 57–64 CrossRef CAS PubMed; (j) E. V. Ilyakina, A. I. Poddel'sky, A. V. Piskunov, N. V. Somov, G. A. Abakumov and V. K. Cherkasov, Inorg. Chim. Acta, 2013, 394, 282–288 CrossRef CAS PubMed; (k) E. V. Ilyakina, A. I. Poddel'sky, A. V. Piskunov, G. K. Fukin, V. K. Cherkasov and G. A. Abakumov, Z. Anorg. Allg. Chem., 2012, 638, 1323–1327 CrossRef CAS PubMed.
- (a) G. A. Abakumov, V. A. Tsaryapkin, L. V. Gorbunova, V. K. Cherkasov and G. A. Razuvaev, Russ. Chem. Bull., 1981, 30, 157–161 CrossRef; (b) G. A. Razuvaev, G. A. Abakumov, P. Y. Bayushkin, V. A. Tsaryapkin and V. K. Cherkasov, Russ. Chem. Bull., 1984, 33, 1915–1922 CrossRef; (c) M. A. Brown, B. R. McGarvey, A. Ozarowski and D. G. Tuck, J. Organomet. Chem., 1998, 550, 165–172 CrossRef CAS; (d) A. V. Piskunov, I. N. Meshcheryakova, E. V. Baranov, G. K. Fukin, V. K. Cherkasov and G. A. Abakumov, Russ. Chem. Bull., 2010, 59, 361–370 CrossRef CAS.
- A. V. Piskunov, A. V. Maleeva, I. N. Meshcheryakova and G. K. Fukin, Russ. J. Coord. Chem., 2013, 39, 245–256 CrossRef CAS.
- (a) A. V. Piskunov, I. N. Mescheryakova, A. S. Bogomyakov, G. V. Romanenko, V. K. Cherkasov and G. A. Abakumov, Inorg. Chem. Commun., 2009, 12, 1067–1070 CAS; (b) A. V. Piskunov, I. N. Mescheryakova, G. K. Fukin, V. K. Cherkasov and G. A. Abakumov, New J. Chem., 2010, 34, 1746–1750 RSC.
- Y. Lure, Handbook of Analytical Chemistry, Khimia, Moscow, 1971, p. 454 Search PubMed.
- J. Emsley, The Elements, Clarendon Press, Oxford, 1991, p. 251 Search PubMed.
- S. S. Batsanov, Russ. J. Inorg. Chem., 1991, 36, 1694–1706 Search PubMed.
- S. N. Brown, Inorg. Chem., 2012, 51, 1251–1260 CrossRef CAS PubMed.
- (a) I. A. Guzei and M. Wendt, Dalton Trans., 2006, 3991–3999 RSC; (b) I. A. Guzei and M. Wendt, Program Solid-G, Madison, WI, USA, 2004 Search PubMed.
- (a) G. K. Fukin, I. A. Guzei and E. V. Baranov, J. Coord. Chem., 2007, 60, 937–944 CrossRef CAS; (b) G. K. Fukin, I. A. Guzei and E. V. Baranov, J. Coord. Chem., 2008, 61, 1678–1688 CrossRef CAS; (c) G. K. Fukin, I. A. Guzei, E. V. Baranov and G. A. Domrachev, Struct. Chem., 2009, 20, 643–654 CrossRef CAS; (d) G. K. Fukin, E. V. Baranov, A. I. Poddel'sky, V. K. Cherkasov and G. A. Abakumov, ChemPhysChem, 2012, 13, 3773–3776 CrossRef CAS PubMed.
- D. D. Perrin, W. L. F. Armarego and D. R. Perrin, Purification of Laboratory Chemicals, Pergamon, Oxford, 1980 Search PubMed.
- B. H. Freeland and D. G. Tuck, Inorg. Chem., 1976, 15, 475–476 CrossRef CAS.
- J. S. Poland and D. G. Tuck, J. Organomet. Chem., 1972, 42, 315–323 CrossRef CAS.
- L. C. Carvalho, M. M. Oliveira, C. Peppe, G. M. Pessoa, A. G. Souza and C. Airoldi, Thermochim. Acta, 1999, 328, 223–230 CrossRef.
- Bruker, SAINTPLUS Data Reduction and Correction Program v.6.02a, Bruker AXS, Madison, WI, USA, 2000 Search PubMed.
- G. M. Sheldrick, SADABS v.2.01, Bruker/Siemens Area Detector Absorption Correction Program, Bruker AXS, Madison, WI, USA, 1998 Search PubMed.
- G. M. Sheldrick, SHELXTL v.6.12, Structure Determination Software Suite, Bruker AXS, Madison, WI, USA, 2000 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Rob, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. AllLaham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, Revision A.1, Gaussian, Inc., Pittsburgh (PA), 2003 Search PubMed.
Footnote |
| † CCDC 1002475–1002477 for compounds 5–7. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra05408c |
| This journal is © The Royal Society of Chemistry 2014 |