Large-scale production of fine-sized Zn2SiO4:Mn phosphor microspheres with a dense structure and good photoluminescence properties by a spray-drying process†
Jung Sang Choa,
Su Min Leeb,
Kyeong Youl Jungc and
Yun Chan Kang*a
aDepartment of Materials Science and Engineering, Korea University, Anam-dong, Seongbuk-gu, Seoul 136-713, Republic of Korea. E-mail: yckang@korea.ac.kr; Fax: +82 2 928 3584
bDepartment of Chemical Engineering, Konkuk University, 1 Hwayang-dong, Gwangjin-gu, Seoul 143-701, Republic of Korea
cDepartment of Chemical Engineering, Kongju National University, 275 Budae-Dong, cheonan, Chungnam 330-717, Republic of Korea
First published on 5th September 2014
Abstract
In this work, a spray-drying method was used to prepare densely structured Zn2SiO4:Mn phosphor particles. Several organic materials such as citric acid, sucrose, and dextrin were examined as additives to control the particle morphology. Consequently, dextrin was found to be the most effective organic additive for obtaining dense precursor powders after the drying process. After the heat-treatment process, the dextrin-assisted precursor particles were turned into dense spherically shaped particles with a mean particle size of 1.3 μm and the high crystallinity of a pure Zn2SiO4 phase. The luminescence properties were changed by changing the types of organic additives. The phosphor particles with the smallest surface area and the powder with the largest crystallite size showed the highest luminescence intensity. Finally, the dense spherical particles obtained from the spray solution containing dextrin had an emission intensity corresponding to 176% that of the phosphor powders prepared from the solution without the organic additive.
Introduction
Manganese-doped zinc silicate (Zn2SiO4:Mn2+) is an efficient green phosphor widely used in lamps, electroluminescent (EL) devices, cathode ray tubes (CRTs), medical imaging radiation detectors, and plasma displays (PDPs) owing to its chemical stability and high luminescent efficiency. It also has a highly saturated color.1–4As far as the synthesis process is concerned, commercial Zn2SiO4:Mn2+ phosphor powders have been produced by solid-state reactions (SSRs) using solid precursors (e.g., ZnO, SiO2, and MnCO3) at high reaction temperatures in order to obtain highly crystalline powders.5,6 Despite the simplicity of this method, the resulting phosphor particles have an irregular morphology and wide size distribution between 2 and 20 μm. Also, the generation of an impurity phase owing to the inhomogeneous mixing of the solid precursors is sometimes inevitable. Thus, a long reaction time or a special additive such as flux in the SSR process is required in order to obtain high-purity powders. Furthermore, the SSR method requires additional steps such as ball milling and washing in order to obtain fine-sized particles.
Recent technological advances in display devices require fine-sized, dense phosphor particles with a narrow size distribution for the improved photoluminescent property.7–10 Thus, the development of new synthetic techniques for large-scale production of Zn2SiO4:Mn2+ phosphor powders with the required size, size distribution, and luminescence has received significant attention in the area of luminescent research. For this reason, a number of synthetic approaches for the preparation of Zn2SiO4:Mn2+ have been proposed, including sol–gel,11–14 combustion,4 polymer precursor,15 glycothermal,1 spray pyrolysis,16–18 and hydrothermal methods.2,19–23
The spray-drying approach has been widely applied to the ceramics industry for producing a dried powder from a liquid solution or suspension.24–31 In the conventional spray-drying method, the homogeneous precursor solution or colloidal suspension is atomized into small droplets and simultaneously injected into a hot chamber for the drying process. The resultant powders are then thermally treated until the desired phase is formed. The process is simple and cost effective, and it can be easily scaled up.32 However, the powders obtained typically show hollow and shell-like aggregates with submicron-sized primary particles because of the solute concentration gradient during the drying step.30,31 Up to now, to the best of our knowledge, the production of densely structured phosphor powders through the spray-drying approach has not been reported. Therefore, it is challenging to develop a new way for the large-scale production of fine-sized, non-agglomerated, and densely structured Zn2SiO4:Mn2+ phosphor powders.
In the present study, a simple and scalable synthetic method for the preparation of filled-structured Zn2SiO4:Mn2+ phosphor powder is introduced. The spray-drying system was used to prepare a metal salts–organic composite as an intermediate precursor from a solution containing an organic additive. Subsequently, densely structured phosphor particles were obtained after a simple heat-treatment process. Sucrose, citric acid, and dextrin were examined as organic additives because they have been used as a carbon source in the preparation of functional materials.33–38 The effect of the specific organic additives used in the preparation of the intermediate precursor powders on the morphology and photoluminescent intensity of Zn2SiO4:Mn2+ powders was investigated in detail.
Experimental
Sample preparation
Zn1.9SiO4:Mn0.1 (Zn2SiO4:Mn) powders were prepared via a spray-drying system. Fig. S4† shows the schematic illustration and photograph of the spray-drying system. For the synthesis of the powders, starting solutions (0.2 M) were prepared by adding 38.4 g zinc nitrate hexahydrate [Zn(NO3)2·6H2O, 98%, Aldrich], 4.0 g silica (SiO2, 99.8%, Aldrich), and 1.7 g manganese acetate tetrahydrate [Mn(CH3COO)·4H2O, 99%, Aldrich] as Zn, Si, and Mn source materials into 1 L of deionized water, respectively. Next, the organic additives, citric acid monohydrate (CA, C6H10O8, Junsei), sucrose (C12H22O11, Junsei), and dextrin [(C6H10O5)n, n = 10–200, Samchun] were added into the above solutions. The amount of citric acid monohydrate and sucrose was fixed at 50 g L−1 in the solutions, whereas the amount of dextrin added to the solutions was varied at 10, 50, and 100 g L−1. The temperatures at the inlet and outlet of the spray dryer were 250 °C and 120 °C, respectively. A two-fluid nozzle was used as an atomizer, and the atomization pressure was 240 kPa. The precursor powders obtained by the spray drying of the spray solution were post-heat treated at 1100 °C for 3 h at a heating rate of 5 °C min−1 in order to accomplish the decomposition of the organic additive and subsequent crystallization. Thereafter, the powders were reduced at 775 °C for 1 h at a heating rate of 5 °C min−1 under a reducing atmosphere (5% H2/Ar mixture gas) to activate the manganese dopant.Characterizations
The microstructures of the powders were observed by scanning electron microscopy (SEM; JSM-6060, JEOL). The crystal phases of the powders were evaluated by X-ray diffractometer (XRD; X'Pert PRO MPD, Philips) using Cu Kα radiation (λ = 1.5418 Å). The surface areas of the powders were measured by the Brunauer–Emmett–Teller (BET) method using N2 as the adsorbate gas. Thermogravimetric (TG) analysis was performed using a thermogravimetric analyzer (Pyris 1 TGA, Perkin Elmer) in the temperature range of 25–650 °C at a heating rate of 10 °C min−1 under a static air atmosphere. The photoluminescence spectra for the samples of about 0.2 g were measured by a spectrophotometer (LS 50, Perkin Elmer) using a Xe flash lamp as an excitation light source. An image analyzer (ImageJ, NIH) was used to determine the particle-size distribution of the powders.Results and discussion
Scheme 1 describes the formation mechanism of the fine sized dense Zn2SiO4:Mn phosphor microsphere. The commercially applicable spray drying process is used for the preparation of metal salt–dextrin composite as an intermediate precursor from an aqueous spray solution. Dextrin plays a key role in the drying stage of the droplet to control the morphology of the spray-dried powder. The drying of dextrin within the droplet forms a highly viscous gel consisting of a three-dimensional polymer network. This viscous gel promotes volume precipitation and results in the formation of spherical-shaped metal salt–dextrin composite powder with dense structure. Finally, the dextrin-assisted intermediate precursor is turned into spherically shaped dense Zn2SiO4:Mn powder after a simple heat-treatment process.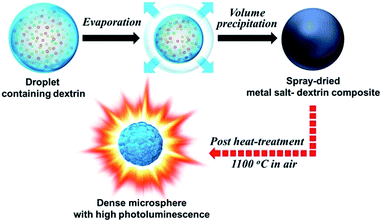 | ||
| Scheme 1 Schematic diagram of formation mechanism of the fine sized dense Zn2SiO4:Mn phosphor microspheres by spray drying process. | ||
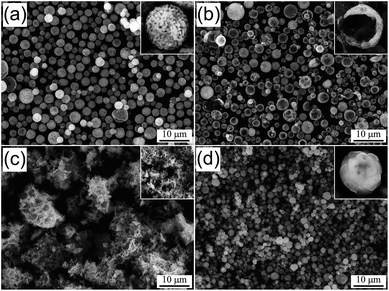
The morphologies of the spray-dried precursor powders prepared from the spray solution without and with various organic additives are shown in Fig. 1. In Fig. 1a, the powders prepared from the solution without an organic additive show a loosely packed structure with spherically shaped particles. When using CA as an organic additive, the obtained powders also had spherically shaped particles but a hollow structure, as shown in the inset image of the crushed powder in Fig. 1b. However, when using sucrose, the spray-dried powders melted to form an aggregated structure by absorbing water molecules from the surrounding environment, as shown in Fig. 1c. In contrast, the use of the spray solution with dextrin made it possible to produce dense and aggregation-free powders; the particles were completely spherical with an average particle size of 3.3 μm. From these results, dextrin was determined as the most efficient organic additive for developing a dense precursor powder in the spray drying process.
 | ||
| Fig. 1 SEM images of the spray-dried precursor powders prepared from the solutions (a) without organic additive, and with (b) CA, (c) sucrose, and (d) dextrin. | ||
The Zn2SiO4:Mn phosphor powders prepared directly by spray drying had no photoluminescence properties owing to the low processing temperature, irrespective of the organic additives. According to the literature, the optimum post-treatment temperature of Zn2SiO4:Mn phosphor powders showing maximum photoluminescence intensity was 1100 °C.39 Therefore, the as-spray-dried phosphor powders were post-treated at 1100 °C and the morphologies of the powders were observed by SEM; the results are shown in Fig. 2. The post-treated powders prepared from the solution without organic additives had spherical particles but a porous structure, as shown in Fig. 2a. The average particle size estimated from the SEM data was approximately 3.1 μm. Meanwhile, when using CA as the organic additive, the post-treated powders had a hollow structure, similar to the case before the thermal treatment (Fig. 2b). The powders prepared from the solution with sucrose were irregularly shaped agglomerates consisting of submicron-sized primary particles (Fig. 2c). As shown in Fig. 2d, when the dextrin was used, dense and spherical particles were obtained and a narrow size distribution ranging from 0.8 to 2.2 μm was observed, as shown in Fig. S1.† The average particle size was approximately 1.3 μm.
 | ||
| Fig. 2 SEM images of the post-treated Zn2SiO4:Mn powders prepared from the solutions (a) without organic additive, and with (b) CA, (c) sucrose, and (d) dextrin. | ||
To confirm the porosity variation with varying organic additives, the nitrogen adsorption–desorption isotherms were measured and the results are displayed in Fig. S2.† The BET surface area is given in the figure. The specific surface areas of the specimens prepared from the solutions containing no additive, CA, sucrose, and dextrin were 18, 13, 7, and 5 m2 g−1, respectively. Clearly, the use of organic additives resulted in a decrease of the surface area as a consequence of particle compaction. When the citric acid was used, the particles were porous, similar to the case in which no organic additive was used. From the BET analysis, the dextrin was confirmed to be the most efficient in terms of preparing dense phosphor particles having a spherical shape.
Fig. 3 shows the XRD patterns of the Zn2SiO4:Mn phosphor powders prepared from the solutions with and without organic additives. All the samples in Fig. 3 were thermally treated at 1100 °C. The observed diffraction peaks are well indexed to a pure hexagonal-structured Zn2SiO4 phase (JCPDS card no. 37-1485) irrespective of whether the organic additives are used or not. The mean crystallite sizes of the post-treated powders were calculated by using the Scherrer equation and the diffraction angle and peak width for the (410) phase in the XRD patterns. The resulting crystallite size of the phosphor particles prepared without the organic additives was 37 nm. The phosphor samples prepared using CA, sucrose, and dextrin had crystallite sizes of 39, 43, and 43 nm, respectively.
 | ||
| Fig. 3 XRD patterns of the post-treated Zn2SiO4:Mn powders prepared from the solutions without carbon source material and with CA, sucrose, and dextrin. | ||
The morphological characteristics of Zn2SiO4:Mn phosphor particles before and after the post-thermal treatment can be affected by the type of organics in the composite precursor powders after spray drying. In terms of the particle morphology and the densification, dextrin was the best additive, and thus the effect of the concentration of dextrin on the morphology of the Zn2SiO4:Mn phosphor particles was studied before and after thermal treatment. To do this, the concentration of dextrin in the spray solution was varied at 10, 50, and 100 g L−1. The morphologies of the powders before and after post-treatment at 1100 °C are shown in Fig. 4. The sphericity and the apparent compactness of the composite precursor powders were affected by the concentration of dextrin in the spray solution. When the concentration of dextrin was 10 g L−1, the precursor particles before thermal treatment had a submicron-sized spherical shape, but a loosely packed hollow structure was generated (Fig. 4a). From the SEM image after the post-thermal treatment, a hollow structure with a fractured shell can be more clearly identified, as shown in Fig. 4c. Fig. 4b shows the SEM image for the precursor powder prepared at a dextrin concentration of 100 g L−1 before the thermal treatment. The apparent compactness of the precursor powder prepared at 100 g L−1 was similar to that of the powders prepared at 50 g L−1 (Fig. 1d), but it was much higher than that of the precursor powder prepared at the dextrin concentration of 10 g L−1. Moreover, the morphology and microstructure of the Zn2SiO4:Mn phosphor powders after the thermal treatment were strongly dependent on the dextrin concentration, as shown in Fig. 4c (10 g L−1), Fig. 2d (50 g L−1), and Fig. 4d (100 g L−1). The post-treated Zn2SiO4:Mn phosphor powders prepared at a dextrin concentration of 100 g L−1 had irregularly shaped aggregates consisting of nanoparticles (Fig. 4d). On the basis of the results achieved so far, there seems to be an optimal quantity of organic additives for the preparation of spherical and dense Zn2SiO4:Mn phosphor particles. That is, the optimal concentration of dextrin was found to be approximately 50 g L−1. For the samples prepared at dextrin concentrations of 10 g L−1 and 100 g L−1, the XRD patterns were measured in order to see the effect of the dextrin concentration on the changes in the crystal phase of the phosphor particles after the post-thermal treatment. As shown in Fig. S3,† a pure hexagonal-structured Zn2SiO4 phase was observed irrespective of the concentration of dextrin added in the spray solution. From this result, it is clear that the dextrin quantity did not change the crystal phase of the final phosphor particles, but it strongly affected the morphology and the microstructure.
 | ||
| Fig. 4 SEM images of the Zn2SiO4:Mn powders prepared from the solution with different concentration of dextrin: (a), (b) as-spray dried precursor powders, and (c), (d) as-thermal treated powders. | ||
Fig. 5 shows the photoluminescence properties of the Zn2SiO4:Mn phosphor powders obtained after post-thermal treatment at 1100 °C. The emission spectrum was measured under an excitation of 254 nm ultraviolet (UV) light, which is attributed to the charge transfer transition of activators.40,41 Under the 254 nm excitation, Zn2SiO4:Mn phosphors are known to have green emission, as shown in Fig. 5b. The prepared phosphor particles show a band emission with a peak of 528 nm. This band emission is assigned to the 4T1 → 6A1 transition of Mn2+.40
According to the excitation and emission spectra, no changes in the peak position and the shape were observed with changing the type of organic additive. The emission and excitation intensity, however, varied greatly according to whether the organic additive was used or not. Also, the luminescence properties were influenced by changing the type of organic additive. It is clear that the use of organic additives made it possible to enhance the luminescence intensity. The emission intensity was in the order of dextrin > sucrose > CA > w/o organic additive. The phosphor powders prepared from the spray solution with dextrin had the highest emission intensity, which was 176% that of the phosphor powders obtained from the spray solution without organic additive. The luminescence properties can be influenced by the crystallinity, crystal phase, morphology, and porosity of the phosphor powders. According to the XRD analysis, no difference in the crystallographic form was observed for any of the samples shown in Fig. 3. However, the morphology, crystallinity, and porosity were different with the different types of organic additives. Large phosphor particles should exhibit higher emission because the crystallinity is thought to be enhanced with increasing crystallite size. With sucrose and dextrin, there was no difference in the crystallite size. However, the emission intensity of the phosphor particles prepared using dextrin was higher than that of those prepared using sucrose. Under the assumption that there is no difference in the particle size and morphology, the sample with the smallest surface area is thought to exhibit the better emission intensity owing to a reduction in the number of surface defects. The surface area of the phosphor powder prepared using dextrin was the smallest, as shown in Fig. S2.† Consequently, when dextrin was used as the organic additive, its large emission intensity is well explained from the fact that the prepared particles have high crystallinity of a pure Zn2SiO4 phase, a spherical shape, and a dense structure.
Conclusions
In this study, a simple and scalable synthetic method for the preparation of densely structured Zn2SiO4:Mn phosphor microspheres is proposed. The particle morphology and microstructure of Zn2SiO4:Mn phosphor particles could be controlled by the organic additive in the spray solution. Among the organic additives used in this study, i.e., citric acid, sucrose, and dextrin, dextrin was the best in terms of preparing high-density particles with a completely spherical shape by reducing the hygroscopicity of the metal salts and developing dense precursor powders during the spray-drying process. Finally, densely structured Zn2SiO4:Mn phosphor powders with a narrow size distribution after the post-thermal treatment process could be obtained by controlling the quantity of dextrin. In terms of the photoluminescence intensity, the powders having high crystallinity and a dense and spherical morphology were better than those having an irregular shape or a highly porous structure. As a result, the spherical and dense particles prepared by using dextrin showed the highest luminescent properties. Therefore, the synthetic method introduced in this study can be applied for large-scale production of Zn2SiO4:Mn phosphor powders with good photoluminescence properties. In addition, the spray-drying process introduced in this study can be widely applied to the preparation of various types of ceramic powders for wide applications.Acknowledgements
“This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (no. 2012R1A2A2A02046367). The TG and XRD analysis were carried out at the Korea Basic Science Institute (Daegu).”Notes and references
- K. Uegaito, S. Hosokawa and M. Inoue, J. Lumin., 2012, 132, 64 CrossRef CAS PubMed.
- M. Takesue, A. Suino, Y. Hakuta, H. Hayashi and R. L. Smith Jr, J. Solid State Chem., 2008, 181, 1307 CrossRef CAS PubMed.
- K. S. Sohn, B. H. Cho and H. D. Park, J. Am. Ceram. Soc., 1999, 82, 2779 CrossRef CAS PubMed.
- R. P. S. Chakradhar, B. M. Nagabhushana, G. T. Chandrappa, K. P. Ramesh and J. L. Rao, J. Chem. Phys., 2004, 121, 10250 CrossRef CAS PubMed.
- A. Morell and N. El Khiati, J. Electrochem. Soc., 1993, 140, 2019 CrossRef CAS PubMed.
- Y. Wang, Y. Hao and L. Yuwen, J. Alloys Compd., 2006, 425, 339 CrossRef CAS PubMed.
- N. Joffin, B. Caillier, J. Dexpert-Ghys, M. Verelst, G. Baret, A. Garcia, P. Guillot, J. Galy, R. Mauricot and S. Schamm, J. Phys. D: Appl. Phys., 2005, 38, 3261 CrossRef CAS.
- Y. C. Kang and H. D. Park, Appl. Phys. A, 2003, 77, 529 CrossRef CAS PubMed.
- Y. C. Kang, H. S. Roh, S. B. Park and K. Y. Jung, Jpn. J. Appl. Phys., 2004, 43, 5302 CrossRef CAS.
- Y. C. Kang, I. W. Lenggoro, S. B. Park and K. Okuyama, J. Solid State Chem., 1999, 146, 168 CrossRef CAS.
- A. Patra, G. A. Baker and S. N. Baker, J. Lumin., 2005, 111, 105 CrossRef CAS PubMed.
- R. Selomulya, S. Ski, K. Pita, C. H. Kam, Q. Y. Zhang and S. Buddhudu, Mater. Sci. Eng., B, 2003, 100, 136 CrossRef.
- Q. Y. Zhang, K. Pita and C. H. Kam, J. Phys. Chem. Solids, 2003, 64, 333 CrossRef CAS.
- T. S. Copeland, B. I. Lee, J. Qi and A. K. Elrod, J. Lumin., 2002, 97, 168 CrossRef CAS.
- K. Su, T. D. Tilley and M. J. Sailor, J. Am. Chem. Soc., 1996, 118, 3459 CrossRef CAS.
- J. S. Lee, M. H. Oh, P. Kumar, A. Khanna, R. K. Singh and M. B. Ranade, J. Therm. Spray Technol., 2011, 20, 1001 CrossRef CAS.
- C. H. Lee, Y. C. Kang, K. Y. Jung and J. G. Choi, Mater. Sci. Eng., B, 2005, 117, 210 CrossRef PubMed.
- S. H. Nam, M. H. Kim, J. Y. Lee, S. D. Lee and J. H. Boo, Funct. Mater. Lett., 2010, 3, 97 CrossRef CAS.
- J. Wan, Z. Wang, X. Chen, L. Mu, W. Yu and Y. Qian, J. Lumin., 2006, 121, 32 CrossRef CAS PubMed.
- T. S. Ahmadi, M. Haase and H. Weller, Mater. Res. Bull., 2000, 35, 1869 CrossRef CAS.
- M. Takesue, K. Shimoyama, S. Murakami, Y. Hakuta, H. Hayashi and R. L. Smith Jr, J. Supercrit. Fluids, 2007, 43, 214 CrossRef CAS PubMed.
- J. H. Zeng, H. L. Fu, T. J. Lou, Y. Yu, Y. H. Sun and D. Y. Li, Mater. Res. Bull., 2009, 44, 1106 CrossRef CAS PubMed.
- X. Yu and Y. Wang, J. Phys. Chem. Solids, 2009, 70, 1146 CrossRef CAS PubMed.
- K. Uematsu, J. Y. Kim, M. Miyashita, N. Uchida and K. Saito, J. Am. Ceram. Soc., 1990, 73, 2555 CrossRef CAS PubMed.
- M. Vicent, E. Sánchez, I. Santacruz and R. Moreno, J. Eur. Ceram. Soc., 2011, 31, 1413 CrossRef CAS PubMed.
- M. Serantoni, A. Piancastelli, A. L. Costa and L. Esposito, Opt. Mater., 2012, 34, 995 CrossRef CAS PubMed.
- G. Jean, V. Sciamanna, M. Demuynck, F. Cambier and M. Gonon, Ceram. Int., 2014, 40, 10197 CrossRef CAS PubMed.
- H. M. Bian, Y. Yang, Y. Wang and W. Tian, Powder Technol., 2012, 219, 257 CrossRef CAS PubMed.
- D. S. Jung, T. H. Hwang, S. B. Park and J. W. Choi, Nano Lett., 2013, 13, 2092 CrossRef CAS PubMed.
- G. Bertrand, P. Roy, C. Filiatre and C. Coddet, Chem. Eng. Sci., 2005, 60, 95 CrossRef CAS PubMed.
- S. J. Lukasiewicz, J. Am. Ceram. Soc., 1989, 72, 617 CrossRef CAS PubMed.
- S. H. Choi and Y. C. Kang, Chem.–Eur. J., 2014, 20, 5835 CrossRef CAS PubMed.
- M. Y. Son, J. H. Kim and Y. C. Kang, Electrochim. Acta, 2014, 116, 44 CrossRef CAS PubMed.
- J. H. Kim and Y. C. Kang, Nanoscale, 2014, 6, 4789 RSC.
- S. H. Choi and Y. C. Kang, Chem.–Eur. J., 2014, 20, 5835 CrossRef CAS PubMed.
- F. Yu, J. J. Zhang, Y. F. Yang and G. Z. Song, J. Solid State Electrochem., 2010, 14, 883 CrossRef CAS PubMed.
- J. Lai, H. Guo, Z. Wang, X. Li, X. Zhang, F. Wu and P. Yue, J. Alloys Compd., 2012, 530, 30 CrossRef CAS PubMed.
- B. Lin, Z. J. Wen and W. X. Han, Solid State Ionics, 2008, 179, 1750 CrossRef CAS PubMed.
- H. Y. Koo, S. H. Lee and Y. C. Kang, Jpn. J. Appl. Phys., 2008, 47, 7407 CrossRef CAS.
- C. Barthou, J. Benoti, P. Benalloui and A. Morell, J. Electrochem. Soc., 1994, 141, 524 CrossRef CAS PubMed.
- A. Morell and N. E. Khiati, J. Electrochem. Soc., 1993, 140, 2019 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06903j |
| This journal is © The Royal Society of Chemistry 2014 |

