Reactivity of nitric oxide with ruthenium complexes derived from bidentate ligands: structure of a ruthenium nitrosyl complex, photoinduced generation and estimation of nitric oxide†
Kaushik Ghosh*,
Rajan Kumar,
Kapil Kumar,
Anand Ratnam and
U. P. Singh
Department of Chemistry, Indian Institute of Technology, Roorkee, Roorkee-247667, Uttarakhand, India. E-mail: ghoshfcy@iitr.ernet.in
First published on 8th September 2014
Abstract
Ruthenium complexes [Ru(L1)(PPh3)2(CO)Cl] (1) [where L1H is (E)-2-((naphthalen-1-ylimino)methyl)phenol] and [Ru(L2)(PPh3)2(CO)Cl] (2) [where L2H is (E)-1-((naphthalen-1-ylimino) methyl) naphthalene-2-ol] [H stands for dissociable proton] were synthesized and characterized by UV-Vis, IR and NMR spectral studies. Nitric oxide reactivity studies afforded ruthenium nitrosyl complexes [Ru(L1)(PPh3)(NO2)(NO)Cl] (1a) and [Ru(L2)(PPh3)(NO2)(NO)Cl] (2a) from complexes 1 and 2, respectively. Complexes 1a and 2a were characterized by different spectroscopic studies. The molecular structure of complex 2a was determined by X-ray crystallography. The redox properties of all the complexes were investigated by cyclic voltammetry. Coordinated nitric oxide was found to be photolabile and photogenerated NO was trapped by reduced myoglobin and liberated NO was estimated by the Griess reaction.
Introduction
Nitric oxide (NO) has been recognized as a signaling molecule and a wide variety of physiological processes like neurotransmission, immune response, blood pressure control, and cellular apoptosis etc. are governed by this small paramagnetic molecule.1 In biosystems, nitric oxide, a highly reactive radical molecule, is produced by an enzyme called nitric oxide synthase (NOS). In recent years there has been an upsurge of interest in the chemistry of nitric oxide and especially the synthesis of nitrosyl complexes that are capable of releasing NO upon irradiation with light.2 Such types of photosensitive complexes are required for applications in photodynamic therapy (PDT).3,4 We have been working in this area and revealed the role of the carbanion in ruthenium(III) cyclometallates.5 Moreover, we also investigated their nitric oxide reactivity and their photophysical properties in other complexes.6This work originated from our recent reports on ruthenium(III) cyclometallates using LH2.5 We designed and synthesized ligands L1H [(E)-2-((naphthalen-1-ylimino)methyl)phenol] and L2H [(E)-1-((naphthalen-1-ylimino)methyl)naphthalene-2-ol] [where H is the dissociable proton] (shown in Scheme 1) with an intention of C–H bond activation (hydrogen of C8) of naphthyl group. Reactions of L1H and L2H with [Ru(PPh3)3Cl2] will be discussed and we report here the synthesis and characterization of ruthenium complexes [Ru(L1)(PPh3)2(CO)Cl] (1), [Ru(L1)(PPh3)(NO2)(NO)Cl] (1a), [Ru(L2)(PPh3)2(CO)Cl] (2) and [Ru(L2)(PPh3)(NO2)(NO)Cl] (2a) (Schemes 2 and 3). Molecular structure of complex 2a was determined by X-ray crystallography. Photolability of the coordinated NO was investigated upon illumination of visible as well as UV light. Estimation of photoreleased NO and trapping of nitric oxide were investigated.
Experimental section
General procedures
All the chemicals used were of reagent grade. Analytical grade reagents naphthalene-1-amine, 2-hydroxybenzaldehyde, sulphanilamide, 2-hydroxy-1-naphthaldehyde, sodium nitrite, naphthylethylenediamine dihydrochloride (NED) (Himedia Laboratories Pvt. Ltd, Mumbai, India), RuCl3·3H2O, triphenylphosphine (SRL, Mumbai, India), disodium hydrogen phosphate anhydrous (RFCL Ltd New Delhi, India) and sodium dihydrogen phosphate (Chemport India Pvt. Ltd Mumbai, India) were used as obtained. Double distilled water was used in all the experiments. Equine skeletal muscle myoglobin was obtained from Sigma Aldrich, Steinheim, Germany.1H and 31P NMR spectra were recorded on Bruker AVANCE, 500.13 MHz spectrometer in the deuterated solvents. Electronic absorption spectra of all the ligands and metal complexes were recorded with Evolution 600, Thermo Scientific UV-Vis spectrophotometer. Infrared spectra were recorded on Thermo Nicolet Nexus FTIR spectrophotometer and were obtained in KBr pellets using 16 scans (in cm−1). Cyclic voltammetric study was performed on a CH-600 electroanalyzer in dichloromethane solution with 0.1 M tetrabutylammonium perchlorate (TBAP) as supporting electrolyte. The working electrode, reference electrode and auxiliary electrode were glassy carbon electrode, Ag/AgCl electrode and Pt wire respectively. The concentration of the compound was ∼10−3 M. The ferrocene/ferrocenium couple appeared at E1/2 = +0.50 V vs. Ag/AgCl (scan rate 0.1 V s−1) in dichloromethane under the same experimental conditions. Chemical actinometry study (with ferrioxalate actinometer) was performed to determine the quantum yield of photoreleased NO.
Synthesis of ligands
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1494, 1397, 1276, 1201, 1151, 975, 757 cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 262 (12
N), 1494, 1397, 1276, 1201, 1151, 975, 757 cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 262 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 394), 352 (10
394), 352 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792). 1H NMR (CDCl3, 500 MHz): δ 13.14 (s, 1H), 8.99 (s, 1H), 8.18 (d, 1H), 7.96 (d, 1H), 7.85 (d, 1H), 7.74 (d, 1H), 7.60–7.54 (m, 3H), 7.46–7.39 (m, 2H), 7.06–7.01 (m, 2H).
792). 1H NMR (CDCl3, 500 MHz): δ 13.14 (s, 1H), 8.99 (s, 1H), 8.18 (d, 1H), 7.96 (d, 1H), 7.85 (d, 1H), 7.74 (d, 1H), 7.60–7.54 (m, 3H), 7.46–7.39 (m, 2H), 7.06–7.01 (m, 2H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1450, 1400, 1303, 1204, 960, 829, 742 cm−1. UV−Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 320 (9878), 385 (12
N), 1450, 1400, 1303, 1204, 960, 829, 742 cm−1. UV−Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 320 (9878), 385 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376). 1H NMR (CDCl3, 500 MHz): δ 15.82 (s, 1H), 9.55 (d, 1H), 8.22 (d, 1H), 7.95–7.79 (m, 4H), 7.65–7.57 (m, 4H), 7.42–7.39 (m, 2H), 7.26–7.24 (m, 1H).
376). 1H NMR (CDCl3, 500 MHz): δ 15.82 (s, 1H), 9.55 (d, 1H), 8.22 (d, 1H), 7.95–7.79 (m, 4H), 7.65–7.57 (m, 4H), 7.42–7.39 (m, 2H), 7.26–7.24 (m, 1H).Synthesis of metal complexes
The precursor complex [Ru(PPh3)3Cl2] was prepared by following the reported procedure.7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1481, 1456, 1434, 765, 696, 523 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 270 (29
N), 1481, 1456, 1434, 765, 696, 523 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 270 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990), 323 (12
990), 323 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658), 440 (4868). 1H NMR ((CD3)2SO, 500 MHz): δ 7.745 (d, 1H), 7.601–7.347 (m, 8H), 7.215–7.081 (m, 27H), 6.804 (t, 1H), 6.735 (d, 1H), 6.541 (d, 1H), 6.441 (d, 1H), 6.081 (m, 2H).
658), 440 (4868). 1H NMR ((CD3)2SO, 500 MHz): δ 7.745 (d, 1H), 7.601–7.347 (m, 8H), 7.215–7.081 (m, 27H), 6.804 (t, 1H), 6.735 (d, 1H), 6.541 (d, 1H), 6.441 (d, 1H), 6.081 (m, 2H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1524, 1428, 744, 691, 516 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 360 (9511), 440 (4450). 31P NMR (CDCl3, 500 MHz): δ 41.10 ppm. 1H NMR (CDCl3, 500 MHz): δ 8.45 (d, 1H), 7.58 (d, 1H), 7.52 (d, 1H), 7.42–7.04 (m, 39H), 6.27–6.25 (m, 1H), 6.19 (d, 1H).
N), 1524, 1428, 744, 691, 516 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 360 (9511), 440 (4450). 31P NMR (CDCl3, 500 MHz): δ 41.10 ppm. 1H NMR (CDCl3, 500 MHz): δ 8.45 (d, 1H), 7.58 (d, 1H), 7.52 (d, 1H), 7.42–7.04 (m, 39H), 6.27–6.25 (m, 1H), 6.19 (d, 1H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1311 (νNO2), 751, 693, 523 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 275 (23
N), 1311 (νNO2), 751, 693, 523 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 275 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 251), 375 (5380). 1H NMR (CDCl3, 500 MHz): δ 8.35 (d, 1H), 7.99–7.80 (m, 8H), 7.64–7.40 (m, 16H), 6.95 (s, 1H), 6.91 (d, 1H).
251), 375 (5380). 1H NMR (CDCl3, 500 MHz): δ 8.35 (d, 1H), 7.99–7.80 (m, 8H), 7.64–7.40 (m, 16H), 6.95 (s, 1H), 6.91 (d, 1H).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1531, 1408, 1326 (νNO2), 751, 693, 523 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 312 (23
N), 1531, 1408, 1326 (νNO2), 751, 693, 523 (νPPh3) cm−1. UV-Vis (CH2Cl2; λmax, nm (ε, M−1 cm−1)): 312 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975), 387 (9940). 31P NMR (CDCl3, 500 MHz): δ 20.92 ppm. 1H NMR (CDCl3, 500 MHz): δ 9.21 (d, 1H), 8.63 (d, 1H), 7.92–7.76 (m, 13H), 7.56–7.32 (m, 14H), 6.89 (d, 1H).
975), 387 (9940). 31P NMR (CDCl3, 500 MHz): δ 20.92 ppm. 1H NMR (CDCl3, 500 MHz): δ 9.21 (d, 1H), 8.63 (d, 1H), 7.92–7.76 (m, 13H), 7.56–7.32 (m, 14H), 6.89 (d, 1H).Griess reagent assay
Griess reagent was prepared by mixing equal volumes of 1% sulphanilamide in 5% orthophosphoric acid and 0.1% naphthylethylenediamine dihydrochloride (NED) in distilled water. The estimation of NO or nitrite (NO2−) ion was measured by observing the increase in the absorbance near 538 nm due to the formation of azo dye. Aqueous solutions of sodium nitrite with different concentrations (5–50 μM) were used to prepare standard curve for the determination of nitrite.5cQuantum yield measurements
Standard ferrioxalate actinometer (0.006 M solution of potassium ferrioxalate in 0.1 N H2SO4) was used to determine the intensity of the UV light (λirr = 365 nm). Quantum yield (ΦNO) of NO photorelease for complexes 1a and 2a were determined from the decrease in its absorption band with λmax near 320 nm when irradiated with the light of a UV lamp (λirr = 365 nm) and was calculated by following the procedure reported earlier.5c The cuvette was kept 3 cm away from the UV source to measure the quantum yields.X-ray crystallography
Orange-red crystal of 2a was obtained by slow evaporation of solution from the complexes in a CH2Cl2–methanol mixture. The X-ray data collection and processing for complex was performed on a Bruker Kappa Apex-II CCD diffractometer by using graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) at 296 K for 2a. Crystal structure was solved by direct method. Structure solutions, refinement and data output were carried out with the SHELXTL program.8,9 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed in geometrically calculated positions and refined using a riding model. Images were created with the DIAMOND program.10Preparation of phosphate buffer and myoglobin (Mb) stock solution
A 50 mM phosphate buffer solution of pH 6.8 was prepared by adding 0.4192 g of NaH2PO4·2H2O and 0.3283 g of anhydrous Na2HPO4 to 50 mL of MilliQ water. The volume of the solution was made 100 mL in volumetric flask. Now to prepare myoglobin stock solution, 5 mg of equine skeletal muscle myoglobin was dissolved in 5 mL of buffer solution (vide infra). 100 μL of myoglobin stock solution was diluted to 1 mL in a quartz cuvette with an optical length of 1 cm and was sealed with a rubber septum.Result and discussion
Syntheses
The complexes [Ru(L1)(PPh3)2(CO)Cl] (1) and [Ru(L2)(PPh3)2(CO)Cl] (2) were synthesized (Scheme 4) by the reaction of [Ru(PPh3)3Cl2] and Schiff base ligands L1H and L2H in methanol with triethylamine (Scheme 1), respectively. Both Complexes 1 and 2 (Scheme 2) were red-brown in colour and isolated in good yield (∼74%). The solvent methanol used during the synthesis in all the reactions was the source of the carbonyl in these complexes.11–13 Complexes 1 and 2 were highly soluble in dichloromethane, benzene, acetonitrile and dimethylformamide but less solubility was found in water. Complexes 1 and 2 were treated with in situ generated NO derived from an acidified nitrite (NaNO2) solution with continuous stirring for 2 h in dichloromethane solution. During reaction, the reddish-brown colour of the solution was converted to a reddish-yellow coloured solution. Ruthenium nitrosyl complexes [Ru(L1)(PPh3)(NO2)(NO)Cl] (1a) and [Ru(L2)(PPh3)(NO2)(NO)Cl] (2a) (Scheme 3) were isolated from reddish-yellow colored solution. Complexes 1a and 2a were highly soluble in organic solvents like dichloromethane, acetonitrile and dimethylformamide but less soluble in water.General properties
All the complexes 1, 1a, 2 and 2a were found to be diamagnetic which was confirmed by 1H and 31P NMR spectral studies (Fig. S1–S6†). 1H NMR spectra of complexes 1, 1a, 2 and 2a indicated their S = 0 ground state in all complexes. In 31P NMR spectrum of 2, we obtained a single peak near 41.11 ppm confirmed the presence of trans PPh3 group13 (Fig. S3†). But in case of 2a, In 31P NMR spectrum we found a peak near 22.15 ppm due to the presence of only one PPh3 group6b (Fig. S6†). The UV-Vis spectra of red brown complexes 1 and 2 were displayed in Fig. S7.† In complexes 1 and 2, we found a common charge transfer band with λmax near 440 nm. On the other hand, complex 1 gave rise to two absorption bands near 270 nm and 323 nm while complex 2 gave rise a absorption band near 360 nm. Both the nitrosyls complexes 1a and 2a were found reddish yellow in color and their electronic spectra were displayed in Fig. S8.† In the electronic spectra of complex 1a, we observed a absorption band near 275 and 365 nm while in case of complex 2a, we found two bands near 312 and 387 nm.The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequencies (νCO) in the IR spectra were observed around 1920 cm−1 which indicated the presence of CO group12,13 in both the complexes 1 and 2 (Fig. S9 and S10†). In the complexes 1a and 2a, the N–O stretching frequencies (νNO) in the IR spectra were found around 1835 cm−1 and 1880 cm−1 respectively5,6a–c (Fig. S11 and S12†) which are expected for {Ru–NO}6 species in nitrosyl complexes.2a The value of νNO was in the range of 1820–1960 cm−1 and a description of {RuII–NO+}6 was proposed for {Ru–NO}6 species2a and these data were also supported by X-ray crystal structure (vide infra). The values of νPPh3 were observed near 750 cm−1, 695 cm−1 and 520 cm−1 for both the complexes 1a and 2a.5,6
O stretching frequencies (νCO) in the IR spectra were observed around 1920 cm−1 which indicated the presence of CO group12,13 in both the complexes 1 and 2 (Fig. S9 and S10†). In the complexes 1a and 2a, the N–O stretching frequencies (νNO) in the IR spectra were found around 1835 cm−1 and 1880 cm−1 respectively5,6a–c (Fig. S11 and S12†) which are expected for {Ru–NO}6 species in nitrosyl complexes.2a The value of νNO was in the range of 1820–1960 cm−1 and a description of {RuII–NO+}6 was proposed for {Ru–NO}6 species2a and these data were also supported by X-ray crystal structure (vide infra). The values of νPPh3 were observed near 750 cm−1, 695 cm−1 and 520 cm−1 for both the complexes 1a and 2a.5,6
Description of molecular structure
The molecular structure of complex [Ru(L2)(PPh3)(NO2)(NO)Cl] (2a) is depicted in Fig. 1. The matrix parameters of complex 2a are described in Table 1 and the selected bond lengths and bond angles are given in Table 2. In the molecular structure of [Ru(L2)(PPh3)(NO2)(NO)Cl] 2a, the phenolato oxygen (O(1)), NO, phosphine group (P(1)), and imine nitrogen (N(9)) constituted the equatorial plane, whereas the Cl(1) and NO2 occupied the trans positions. So, the geometry around the metal centre is found to be distorted octahedral. In the nitrosyl complex 2a, Ru–NNO distance [1.735(16)] was found to be closer to the reported value.6b,14 The N–O distance (1.140) was found to be consistent with the values given in the literature.6b,14b All these data along with Ru–N–O angles (≈177°) in 2a clearly show a {RuII–NO+}6 description of the {Ru–NO}6 moiety.14 Structure solution afforded several important features of this nitric oxide reactivity studies. First, we were unable to get ruthenium cyclometallate via naphthyl hydrogen activation. Second, CO was eliminated during NO coordination. Third, unlike our previous results5,6 ligand nitration was not observed in activated benzene ring having phenolato function. Fourth, nitrite coordination was observed. Fifth, unlike our previous results5 NO was found to be trans to the phenolato function which is rare in the literature.15 Sixth, the PPh3 group was found to be in the same plane of the ligand and NO, however, 31P NMR data clearly indicated trans disposition of PPh3 ligands in complexes 1 and 2. | ||
| Fig. 1 ORTEP diagram (30% probability level) of the complex [Ru(L2)(PPh3)(NO2)(NO)Cl] (2a). Hydrogen atoms are not shown for clarity. | ||
| a GOF = [Σ[w(Fo2 − Fc2)2]/M − N]1/2 (M = number of reflections, N = number of parameters refined).b R1 = Σ||Fo| − |Fc||/Σ|Fo|.c wR2 = [Σ[w(Fo2 − Fc2)2]/Σ[(Fo2)2]]1/2. | |
|---|---|
| Empirical formula | C39H29ClN3O4PRu |
| Formula weight | 771.14 |
| T(K) | 296(2) |
| λ(A)(Mo-Kα) | 0.71073 |
| Crystal system | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a(Å) | 9.136(4) |
| b(Å) | 11.058(4) |
| c(Å) | 18.192(7) |
| α(°) | 102.36(2) |
| γ(°) | 107.76(3) |
| β(°) | 90.79(3) |
| V(Å3) | 1703.5(12) |
| Z | 4 |
| ρcalc (g cm−3) | 1.503 |
| F(000) | 784.0 |
| Theta range(°) | |
| Index ranges | −11 < h < 11, −13 < k < 13, −22 < l < 22 |
| Data/restraints/parameters | 6986/0/430 |
| GOFa on F2 | 1.188 |
| R1b[I > 2σ(I)] | 0.0750 |
| R1[all data] | 0.1384 |
| wR2c [I > 2σ(I)] | 0.1828 |
| wR2 [all data] | 0.2123 |
| Bond lengths (Å) | Bond angles (°) | ||
|---|---|---|---|
| Ru(1)–Cl(1) | 2.394(27) | Ru(1)–N(1)–O(2) | 177.1(6) |
| Ru(1)–O(1) | 1.965(4) | N(1)–Ru(1)–O(1) | 175.70(21) |
| Ru(1)–N(1) | 1.734(6) | N(1)–Ru(1)–N(9) | 95.88(26) |
| Ru(1)–N(9) | 2.078(7) | O(1)–Ru(1)–N(9) | 87.47(21) |
| Ru(1)–N(17) | 2.093(10) | N(1)–Ru(1)–N(17) | 89.98(40) |
| Ru(1)–P(1) | 2.399(2) | O(1)–Ru(1)–N(17) | 87.25(37) |
| N(1)–O(2) | 1.140(13) | N(9)–Ru(1)–P(1) | 172.17(19) |
| N(17)–O(5) | 1.076(22) | N(17)–Ru(1)–Cl(1) | 178.14(32) |
| N(17)–O(6) | 1.106(17) | O(1)–Ru(1)–P(1) | 85.82(16) |
| N(17)–Ru(1)–P(1) | 92.47(29) | ||
Electrochemistry
We have investigated the redox properties of ruthenium center in complexes 1, 2, 1a and 2a by examining their redox properties using cyclic voltammetric studies. The cyclic voltammogram of 1 showed a quasireversible redox couple with an E1/2 value near +0.61 V vs. Ag/AgCl which was assigned to be Ru(II)/Ru(III) couple (Fig. 2(a), red line).6d,16,17 In case of complex 2, we observed a quasireversible redox couple with an E1/2 value of +0.81 V which was assigned to be Ru(II)/Ru(III) couple6d,16,17 and we found also a quasireversible redox couple near −0.60 V vs. Ag/AgCl which was assigned to be Ru(II)/Ru(I) couple (Fig. 2(a), black line).17 We did not observe any redox couple in the case of complex 1a and 2a. Only we observed cathodic peaks and Eca values are found to be at −1.05 V, −0.77 V (for 1a) and −0.92 V, −0.76 V (for 2a) vs. Ag/AgCl (Fig. 2(b)). In the negative potential quasireversible couples (RuIII/RuII) for nitrosyl complexes were reported18,19 by Mascharak and coworkers.Lahiri and coworkers described ligand (nitric oxide) centered reduction of (RuII–NO+)6 → (RuII–NO˙)7 and further is extended to the second one-electron reduction (RuII–NO˙)7 → (RuII–NO−)8 for the appearance of such two types peak at negative potentials.20 The appearance of irreversible cathodic peak clearly expressed that NO-centered reduction to afford a complex having {RuNO}7 species is unfavorable.
Photolysis experiment for nitrosyl complexes
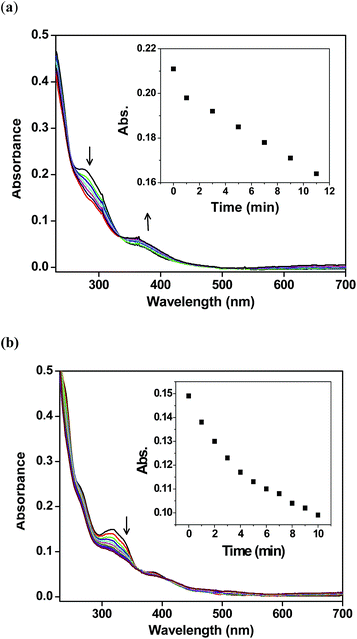
The photolability of coordinated (nitric oxide) NO of ruthenium nitrosyl complexes 1a and 2a was observed under visible as well as UV light in dichloromethane solutions. No change was observed in dark, however, we observed a change in color from reddish-yellow to red brown in the presence of visible light (100 W tungsten lamp) and UV light. Loss of NO was observed when a dichloromethane solutions of 1a and 2a were exposed to low intensity UV light (λmax = 365 nm). In case of complex 1a, the peak intensity decreased near 275 nm and a new peak increased near 365 nm. We found a isobastic point near 332 nm (Fig. 3(a)). For complex 2a, we observed a decreased in peak intensity near 320 nm (Fig. 3(b)). Similarly, illumination of visible light (100 W tungsten lamp), there is a disappearance of peak at 275 nm as well as generation of a new peak near 365 nm for 1a and decreased a peak near 320 nm for 2a. However rapid spectral changes were observed when the solutions were exposed to low intensity light of the UV lamp (λmax = 365 nm). Hence UV light was found to be more efficient in photolytic reaction. The quantum yield (ϕ) values by chemical actinometry for NO photorelease from complexes 1a and 2a (λirr = 365 nm) were found to be 0.003 ± 0.001 and 0.005 ± 0.001 respectively in dichloromethane solutions.Griess reaction to estimate the photoreleased NO
We estimated the amount of photoreleased NO from nitrosyl complexes with the help of Griess reagent assay.5c,6b–e The presence of photolabile NO in complexes 1a and 2a was observed by increasing in the optical density of the produced dye at ∼538 nm in the presence of visible light as well as ultraviolet light. When a solution of a Griess reagent with complexes 1a (50 μM) and 2a (50 μM) was illuminated with visible light (100 tungsten lamp) for 15 minutes then the amount of NO was released ∼3.0 μM and ∼5.0 μM respectively (Table 3). But exposure of UV light to 50 μM solutions of both the complexes 1a and 2a gave rise to ∼6.0 μM and ∼9.0 μM (Fig. 4 and Table 3) of dye formation respectively. The change in absorbance of azo dye produced from Griess reagent was found to be lower under visible light (100 W tungsten lamp). We have compared our data with the data obtained from sodium nitroprusside (SNP), a well known NO donor drug, under the same experimental conditions. In ultraviolet light, 50 μM solution of SNP provided ∼4.0 μM of nitric oxide5c (Table 3). These data afforded the formation of NO in solution and the concentration of photoreleased NO in complex 1a was close to the amount of NO released by SNP however in case of complex 2a, the photorelease NO was found to be better in comparison with the NO released by SNP.Comparing the data for the release of nitric oxide obtained from Griess reagent assay in this report and our other published results,5c,6b,c,e we would like to mention here that complexes having Ru–C bond are better capable of delivering nitric oxide upon light illumination.
NO trapping by reduced myoglobin
The photoreleased NO from the nitrosyl complexes was transferred to the heme iron center of reduced myoglobin using low intensity light of UV lamp. Electronic absorption spectra were obtained in phosphate buffer (pH ∼6.8). For oxidized myoglobin (Mb), we observed an intense band near ∼409 nm (Soret band). The UV-Vis spectra of reduced myoglobin near 433 nm was obtained by addition of excess sodium dithionite to the same cuvette. When acetonitrile solutions of complexes were added to buffer solutions of reduced myoglobin under dark conditions, no reaction was observed. However when the same mixtures were taken under exposure to UV lamp (λ = 365 nm) for 2–3 minutes, the absorption spectra near 421 nm (for 1a, Fig. 5) and 422 nm (for 2a, Fig. S13†) showed the formation of myoglobin–NO adducts.5,6a–cSummary and conclusions
The following are the principal findings and conclusions of the present study.(a) Complexes [Ru(L1)(PPh3)2(CO)Cl] (1) and [Ru(L2)(PPh3)2(CO)Cl] (2) were synthesized from ligands L1H and L2H respectively and the resultant complexes were devoid of Ru–C bond. Hence C–H activation in the naphthyl amine was not achieved.
(b) Reaction of nitric oxide with complexes 1 and 2 afforded ruthenium nitrosyl complexes. Structure solution by X-ray crystallography and spectroscopic data clearly expressed the presence of {Ru–NO}6 moiety and a description of {RuII–NO+}6 was proposed for both complexes 1a and 2a.
(c) Molecular structure revealed few interesting events which were contrary to our previous reports in this nitric oxide reactivity studies. Ligand nitration was not observed, phosphine group was coordinated to the ligand binding plane and nitrite coordination to the metal centre.
(d) Coordinated nitric oxide in complexes 1a and 2a was found to be photolabile under visible as well as low intensity UV light.
(e) Nitric oxide generated after photolytic cleavage of nitrosyl complexes was trapped by reduced myoglobin and the amount of liberated NO in this process was estimated by Griess reaction. Although there is no Ru–C bond in 1a and 2a we compared our data with published results. It has been found out that the ruthenium organometallics are more efficient in terms of photoinduced nitric oxide delivery.
Further experiments on naphthyl C–H bond activation and biological applications on ruthenium-nitrosyl complexes are under progress.
Acknowledgements
KG is thankful to CSIR, India for financial assistance no. 01(2720)/13/EMR-II dated 17-APRIL-2013). RK, KK and AR are thankful to IIT Roorkee for their fellowship.Notes and references
- (a) Nitric Oxide: Biology and Pathobiology, ed. L. J. Ignarro, Academic Press, San Diego, CA, 2000 Search PubMed; (b) M. J. Rose and P. K. Mascharak, Curr. Opin. Chem. Biol., 2008, 12, 238 CrossRef CAS PubMed.
- (a) M. J. Rose and P. K. Mascharak, Coord. Chem. Rev., 2008, 252, 2093 CrossRef CAS PubMed; (b) P. C. Ford, Acc. Chem. Res., 2008, 41, 190 CrossRef CAS PubMed.
- (a) G. B. Richter-Addo and P. Legzdins, Metal Nitrosyls, Oxford University Press, New York, 1992 Search PubMed; (b) G. B. Richter-Addo, P. Legzdins and J. Burstyn, Chem. Rev., 2002, 102, 857 CrossRef CAS PubMed; (c) M. G. Sauaia, R. G. de Lima, A. C. Tedesco and R. S. da Silva, J. Am. Chem. Soc., 2003, 125, 14718 CrossRef CAS PubMed.
- A. K. Patra, R. Afshar, M. M. Olmstead and P. K. Mascharak, Angew. Chem., Int. Ed., 2002, 41, 2512 CrossRef CAS and references therein.
- (a) K. Ghosh, S. Kumar, R. Kumar, U. P. Singh and N. Goel, Inorg. Chem., 2010, 49, 7235 CrossRef CAS PubMed; (b) K. Ghosh, S. Kumar, R. Kumar, U. P. Singh and N. Goel, Organometallics, 2011, 30, 2498 CrossRef CAS; (c) K. Ghosh, S. Kumar, R. Kumar and U. P. Singh, Eur. J. Inorg. Chem., 2012, 929 CrossRef CAS PubMed.
- (a) K. Ghosh, S. Kumar and R. Kumar, Inorg. Chem. Commun., 2011, 14, 146 CrossRef CAS PubMed; (b) K. Ghosh, R. Kumar, S. Kumar and J. S. Meena, Dalton Trans., 2013, 42, 13444 RSC; (c) K. Ghosh, S. Kumar and R. Kumar, Inorg. Chim. Acta, 2013, 405, 24 CrossRef CAS PubMed; (d) K. Ghosh, S. Kumar, R. Kumar and U. P. Singh, J. Organomet. Chem., 2014, 750, 169 CrossRef CAS PubMed; (e) K. Ghosh, S. Kumar and R. Kumar, Eur. J. Inorg. Chem., 2014, 1454 CrossRef CAS PubMed.
- T. A. Stephenson and G. J. Wilkinson, J. Inorg. Nucl. Chem., 1966, 28, 945 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467 CrossRef.
- G. M. Sheldrick, SHELXTL-NT 2000 version 6.12, Reference Manual, University of Gottingen, Pergamon, New York, 1980 Search PubMed.
- B. Klaus, DIAMOND, Version 1.2 c, University of Bonn, Germany, 1999 Search PubMed.
- R. Raveendran and S. Pal, J. Organomet. Chem., 2007, 692, 824 CrossRef CAS PubMed.
- R. Raveendran and S. Pal, J. Organomet. Chem., 2010, 695, 630 CrossRef CAS PubMed.
- J. Xiang, L. T.-L. Lo, C.-F. Leung, S.-M. Yiu, C.-C. Ko and T.-C. Lau, Organometallics, 2012, 31, 7101 CrossRef CAS.
- (a) M. J. Rose and P. K. Mascharak, Inorg. Chem., 2009, 48, 6904 CrossRef CAS PubMed; (b) A. K. Patra, M. J. Rose, K. A. Murphy, M. M. Olmstead and P. K. Mascharak, Inorg. Chem., 2004, 43, 4487 CrossRef CAS PubMed.
- T. A. Heinrich, G. V. Poelhsitz, R. I. Reis, E. E. Castellano, A. Neves, M. Lanznaster, S. P. Machado, A. A. Batista and C. M. Costa-Neto, Eur. J. Med. Chem., 2011, 46, 3616 CrossRef CAS PubMed.
- C. GuhaRoy, S. S. Sen, S. Dutta, G. Mostafa and S. Bhattacharya, Polyhedron, 2007, 26, 3876 CrossRef CAS PubMed.
- K. N. Kumar, R. Ramesh and Y. Liu, J. Inorg. Biochem., 2006, 100, 18 CrossRef CAS PubMed.
- A. K. Patra and P. K. Mascharak, Inorg. Chem., 2003, 42, 7363 CrossRef CAS PubMed.
- M. J. Rose, A. K. Patra, E. A. Alcid, M. M. Olmstead and P. K. Mascharak, Inorg. Chem., 2007, 46, 2328 CrossRef CAS PubMed.
- S. Maji, B. Sarkar, M. Patra, A. K. Das, S. M. Mobin, W. Kaim and G. K. Lahiri, Inorg. Chem., 2008, 47, 3218 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1002231. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra04767b |
| This journal is © The Royal Society of Chemistry 2014 |