Molecularly imprinted TiO2 hybridized magnetic Fe3O4 nanoparticles for selective photocatalytic degradation and removal of estrone
Shoufang Xuab,
Hongzhi Lua,
Lingxin Chen *b and
Xiaochuan Wang*c
*b and
Xiaochuan Wang*c
aSchool of Chemistry and Chemical Engineering, Linyi University, Linyi 276005, China. E-mail: xshfang1981@163.com
bKey Laboratory of Coastal Zone Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China. E-mail: lxchen@yic.ac.cn; Fax: +86 535 2109130; Tel: +86 535 2109130
cKey Laboratory of Ecological Geochemistry, Ministry of Land and Resources, National Research Center for Geoanalysis, Beijing 100037, China. E-mail: wangwxc@iccas.ac.cn; Tel: +86 10 68999579
First published on 15th September 2014
Abstract
As a widely used photocatalyst, titania (TiO2) enjoys significant advantages; however, it faces the severe challenge of poor selectivity. And interestingly, molecular imprinting gains great popularity, owing to its desired recognition specificity. Herein, by using estrone as a template molecule, we prepared molecularly imprinted TiO2 hybridized magnetic ferroferric oxide (Fe3O4) nanoparticles through a semi-covalent approach by a liquid phase deposition method, for selective photocatalytic degradation and removal of target estrone with the irradiation of UV light. The obtained Fe3O4@SiO2@imprinted TiO2 displayed high adsorption capacity, fast kinetics and high selectivity. In the presence of 10 times of coexisting nontarget compounds, the apparent rate constant, kapp, for photodegradation of target estrone over the hybridized nanoparticles, was about 6 times of that over net TiO2. Also, excellent stability during long-time photocatalysis was exhibited. More importantly, the Fe3O4@imprinted TiO2 provided potential application prospectives for photocatalytic removal of trace target organic pollutants in the presence of high-level pollutants.
1. Introduction
With the fast development of industrialization, more and more pollutants are poured into the natural environment, which results in increasingly serious environmental pollution, especially water pollution. Among the organic pollutants, estrone, bisphenol A, tetrabromobisphenol A and 4-nonylphenol etc., with similar structural features and estrogenic activity, are classified as phenolic environmental estrogens (PEEs).1 Because PEEs are fat-soluble and can easily be enriched in the human body, they have become the focus of attention in recent years. As one of the naturally occurring PEEs, estrone has carcinogenic properties and adverse environmental effects.2 Various methods, such as physicochemical treatment,3 biological degradation,4 and photocatalytic degradation5 have been developed to remove organic pollutants in the aquatic environment. Among those methods, photocatalytic degradation using titania (TiO2) has been well studied for environmental protection. Nevertheless, TiO2 photocatalysts possess two severe defects: first, it is as yet difficult to realize selective removal of harmful low-level pollutants in the presence of high-level less harmful pollutants because of the poor selectivity; second, TiO2 nanoparticle is difficult to reuse because of small particle size. Several approaches have been proposed to enhance the selectivity of TiO2. One approach is to control the surface's electric charge by adjusting pH:6 a lower pH is helpful for the degradation of negatively charged contaminants, and a higher pH is favorable for the degradation of positively charged contaminants. The other way is to modify the surface of TiO2 with specific molecules which can obtain a selective and effective adsorption.7 In recent years, molecularly imprinted polymers (MIPs), owing to their attractive selectivity, have been introduced to improve the selectivity of the TiO2 photocatalyst.8–10Commonly, MIPs are prepared by copolymerization of functional monomers and cross-linkers in the presence of target analytes which act as template molecules. After removal of template, recognition sites complementary in size, shape, and functionality to the template are formed in the three-dimensional polymer network.11,12 MIPs as versatile materials have aroused extensive attention for diverse species in various fields.11–15 Coating MIPs on the surface of TiO2 to improve the selectivity of catalytic degradation is an important aspect.8–10 Nowadays, three main methods have been proposed to prepared MIPs coated TiO2 photocatalyst. The first one involves coating organic MIPs layer on TiO2 particles, in which TiO2 acts as photocatalyst, and the layer of MIPs shows specific affinity toward target compounds.9 However, this approach involves a multistep procedure, and organic MIPs would be destroyed after longtime photocatalysis. To avoid such drawbacks, some studies are focused on coating inorganic MIPs on the surface of TiO2 by sol–gel process. For example, Shen et al. prepared Al3+-doped diethyl phthalate molecularly imprinted TiO2@SiO2 nanocomposite particles.10 However, TiO2 were embedded into highly cross-linked MIPs which resulted in relatively low photocatalytic degradation ability. It can be hypothesized that the selective photocatalytic degradation ability of TiO2 would be greatly increased if TiO2 film acts as both photocatalyst and imprinted recognition layer. So, directly imprinting TiO2 might be an efficient way, which can be achieved by liquid phase deposition (LPD) method.16
LPD is known as a soft-solution process for direct preparation of metal oxide thin film on various substrates from aqueous solutions, by means of a ligand-exchange equilibrium reaction between the metal–fluoro complex ions and metal oxide.16 The addition of boric acid as a scavenger for F− ions can shift the chemical equilibrium, resulting in the deposition of metal oxide homogeneously onto substrates.16 Using (NH4)2TiF6 as the precursor, TiO2 films would be deposited. LPD method has been used for protein imprinting17–19 and selective photocatalysis by molecular imprinted TiO2 thin films16 because of its significant advantages especially the mild reaction condition.
In the present work, we proposed to prepare new molecularly imprinted TiO2 photocatalyst for highly selective photodegradation and removal of estrone by LPD on the surface of magnetic ferroferric oxide (Fe3O4) nanoparticles. Fe3O4 was introduced for its magnetic separation ability, SiO2 film was coated on the surface of Fe3O4 nanoparticles beneficial for immobilizing target estrone, and LPD was employed for the formation of footprint cavities on the TiO2 film. With the irradiation of UV light, the obtained Fe3O4@SiO2@imprinted TiO2 could selectively adsorb and photodegrade estrone in aqueous media. The properties of prepared photocatalyst, including molecular binding capacity, recognition specificity and degradation mechanism/kinetics, were investigated in detail. By combining the separation ability of Fe3O4 particles with the functional capability of MIPs and TiO2, the photocatalyst supply perspective applications to simple, rapid, high-efficiency degradation and removal of trace targeted pollutants in the presence of high level non-target pollutants.
2. Experimental
2.1. Materials and apparatus
FeCl3·6H2O, ethylene glycol, NaAc and polyethylene glycol for preparation of magnetic Fe3O4 were obtained from Shanghai Chemical Reagent Company. Dibutyltin dilaurate (DBDU), 3-isocyanatopropyltriethoxysilane (IPTS) and tetraethoxysilicane (TEOS) for modification of Fe3O4 were supplied also by Shanghai Chemical Reagent Company. P25 TiO2 nanoparticles (anatase, hydrophilia) (P25), ammonium hydroxide (25%), hexafluorotitanate (NH4)2TiF6, boric acid, estrone, bisphenol A (BPA) and phenol were provided by Tianjin Chemical Reagent Co., Ltd. Tetrahydrofuran (THF, 99%, Tianjin Jiangtian Chemicals, China) was refluxed over sodium and then distilled. All the chemicals were of analytical reagent grade and used as received without further purification except specified.Morphological observation of nanoparticles was performed by transmission electron microscopy (TEM; JEOL, JEM-1230, Japan). The surface diffractogram of the deposited TiO2 was characterized by X-ray diffraction on an X'Pert PRO X-ray diffractometer with a Cu Ka radiation source (XRD, PANalytical, Netherlands). A FT-IR spectrometer (Thermo Nicolet Corporation, USA) was employed to examine the infrared spectra of samples using a pressed KBr tablet. Brunauer–Emmett–Teller (BET) surface area was determined by nitrogen adsorption/desorption at 77 K using a Micromeritics ASAP 2020 Sorptometer (Micromeritics, ASAP 2020, USA). The samples were degassed in a vacuum at 180 °C prior to adsorption measurements. UV-visible spectra were obtained by a Thermo Scientific NanoDrop 2000/2000c spectrophotometer (Thermo, USA) supplied with a quartz cell. The concentrations of each pollutant in the mixture were analyzed by HPLC equipped with a C18 ODS column (250 mm × 4.6 mm, 5 μm) and DAD detector (Elite Instrument Inc., China).
2.2. Preparation and modification of Fe3O4
Fe3O4 microspheres were synthesized by solvothermal method as reported.20 Fe3O4 modified with IPTS were prepared according to the previous method with a little modification.21 Briefly, 0.10 g of Fe3O4 particles were treated with 50 mL 0.1 M HCl aqueous solution by ultrasonication for 10 min, then separated and washed with deioned water. After homogeneously redispersed in the mixture of 80 mL of ethanol, 20 mL of deioned water and 1.0 mL of ammonia aqueous solution (28 wt%), 2 mL of TEOS was added followed by stirring at room temperature for 6 h. And then1 mL of IPTS was added, and the solution was further reacted for 18 h at room temperature. Then Fe3O4@SiO2 microspheres modified with IPTS were separated and washed with ethanol and water.2.3. Preparation of estrone imprinted TiO2 layer on Fe3O4
Before deposition TiO2 layer on the surface of Fe3O4@SiO2, template estrone was first immobilized on the surface of Fe3O4@SiO2 by the reaction of isocyanate group of IPTS and phenol moiety of estrone, forming a thermally cleavable urethane bond as reported.22 The urethane bond is stable at room temperature, but reversible cleavage occurs at elevated temperatures. Briefly, 0.2 g Fe3O4@SiO2 modified with IPTS was dispersed in 50 mL of dried THF. To the solution, 5 mmol estrone was added at room temperature, followed by the addition of 0.3 mL DBDU, and then the reaction mixture was refluxed for 24 h under nitrogen atmosphere. After reaction, magnetic particles were separated and washed with ethanol.LPD solution was prepared by dissolving ammonium hexafluorotitanate (0.15 mmol) and boric acid (0.3 mmol) into 1 mL deionized water. Then ammonia aqueous solution was added to adjust the pH to 7.0. The LPD solution (1 mL), P25 TiO2 nanoparticles (0.04 g L−1) were added to 5.0 mL of 1.0 mg mL−1 Fe3O4 particles immobilized with estrone water solution, and then the mixture was mildly shaken for 3 h at 25 °C. The resultant magnetic particles were washed several times with deionized water. Subsequently, the imprinted estrone was removed by calcination at 400 °C, which produced the molecularly imprinted TiO2 hybridized Fe3O4 nanoparticles (Fe3O4@SiO2@imprinted TiO2), that is, MIPs for simplicity. As control materials, the non-imprinted polymers (NIPs) were also prepared as described above but without the addition of template estrone.
2.4. Binding property studies of the MIPs
Molecular binding properties of the MIPs including static adsorption, kinetic adsorption and selective binding capacity were estimated by a batch procedure method. The procedures were carried out as follows. MIPs particles with the mass of 30 mg were dispersed in 5 mL of estrone solutions of various concentrations. After shaking at room temperature for 24 h, the samples were separated, and the concentrations of which were determined using HPLC-DAD. HPLC conditions employed for estrone were as follows: mobile phase, methanol–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v); flow rate, 1.0 mL min−1; room temperature; DAD detection, at 280 nm; injection volume, 20 μL. The calibration curves for the detection of estrone was obtained by performing a linear regression analysis ranging from 2.0 to 75 mg L−1. Good linearity was obtained with correlation coefficients of R > 0.99. The limits of detection (LOD) were 1.5 ng based on a signal-to-noise ratio of 3. Precision calculated in terms of intraday repeatability (n = 6) and interday reproducibility (6 different days) was 2.4% and 3.5% respectively.
20, v/v); flow rate, 1.0 mL min−1; room temperature; DAD detection, at 280 nm; injection volume, 20 μL. The calibration curves for the detection of estrone was obtained by performing a linear regression analysis ranging from 2.0 to 75 mg L−1. Good linearity was obtained with correlation coefficients of R > 0.99. The limits of detection (LOD) were 1.5 ng based on a signal-to-noise ratio of 3. Precision calculated in terms of intraday repeatability (n = 6) and interday reproducibility (6 different days) was 2.4% and 3.5% respectively.
The binding amount of estrone was determined by subtracting the residual amount of estrone in solution from the total estrone amount. Meanwhile, the binding kinetics was tested by monitoring the temporal amount of estrone in the solutions. Selectivity experiments were carried out by using BPA and phenol as structural analogs.
2.5. Photocatalytic degradation experiments
The photocatalytic degradation experiments were performed in a 250 mL of ringent photoreactor with stirring at room temperature, in which 40 mL of 10 mg L−1 estrone water solution and 100 mg of photocatalysts were added. A circular 20 W UV light with an emission peak at 254 nm was horizontally positioned 10 cm above the surface of the solution. After immersion for 6 h to achieve the adsorption–desorption equilibrium, the concentrations of the pollutant were determined as the initial concentration, and then the photo-irradiation was started. At different time intervals, 2 mL aliquots were sampled to detect the pollutants.3. Results and discussion
3.1. Preparation and characterization of the estrone imprinted Fe3O4@SiO2@TiO2 (MIPs)
The widely adopted technique for preparing MIPs is non-covalent approach, in which aprotic and low polar organic solvents are often used. However, the non-covalent imprinted polymers often show poor recognition ability for the target in aqueous environment because the hydrogen bond formed between template and functional monomer can be disturbed by the polar solvent. In this work, LPD method involved in aqueous solution was employed for estrone imprinting, and the obtained MIPs were used to remove organic pollutants in aqueous environment. In order to achieve ideal molecular imprinting in aqueous environment, semi-covalent approach23 was adopted.The synthesis procedure for estrone MIPs by semi-covalent approach is illustrated in Scheme 1A, which involves synthesis of Fe3O4 magnetic nanoparticles, silica-shell coating, template immobilization, TiO2 imprinted film deposition and template removal. Hydrophilic Fe3O4 nanoparticles with uniform morphology and high magnetization were prepared by solvothermal method. Then SiO2 shell with isocyanate group was coated on the surface of Fe3O4 by sol–gel process beneficial for immobilization of template. Template estrone was immobilized on the surface of Fe3O4 by covalent urethane bond formed between isocyanate group of IPTS and phenol moiety of estrone. Then TiO2 film was deposited on the surface of Fe3O4 and calcination was used for removing template to form imprinted recognition sites. For the pure semi-covalent imprinting method, template estrone was only immobilized on the surface of Fe3O4@SiO2, and the thickness of the TiO2 shell is about 40 nm (Fig. 1), which resulted in low binding capacity and mass transfer rate. So in the improved scheme, template also was added in the liquid phase deposition solution, and recognition sites can be formed in the whole TiO2 layer, as illustrated in Scheme 1A. The binding capacity and mass transfer can be enhanced in this improved scheme. The reason of choosing TiO2 as the molecularly imprinted layer was that TiO2 can achieve photocatalytic degradation of pollutant. Furthermore, the LPD method is suitable for imprinting because of its mild reaction conditions. Calcination was used to remove the template because reversible cleavage of the urethane bond formed between an isocyanate and a phenol occurs at elevated temperatures.23
TEM images of Fe3O4, Fe3O4@SiO2 and imprinted Fe3O4@SiO2@TiO2 were shown in Fig. 1. All samples displayed spherical shape. Fig. 1A shows the uncoated Fe3O4 microsphere displayed relatively uniform size distribution with the diameter about 400 nm. Fig. 1B shows the Fe3O4 microspheres were fully coated by silica shell with thickness about 10 nm. Fig. 1C clearly shows the uniform core-shell structure of Fe3O4@SiO2@TiO2, and the thickness of the TiO2 shell was about 40 nm. So, it can be confirmed from TEM pictures that TiO2 layer was successfully deposited on the surface of Fe3O4.
The preparation procedure was also estimated by FT-IR, as shown in Fig. 2. Compared with Fe3O4 (a), the Fe3O4@SiO2 (b) and Fe3O4@SiO2–estrone (c) clearly displayed the characteristic peaks of stretch vibration of Si–O–Si (the strong peaks at 1090 cm−1), indicating the formation of silica film. The peaks at 1718 cm−1 ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and 1636 cm−1 ascribed to amine groups (c) indicated the formation of urethane bonds between Fe3O4@SiO2 and estrone, suggesting that the template estrone was immobilized on the surface of Fe3O4@SiO2 particles. After deposited TiO2 film (d), the strong peaks at 1090 cm−1 disappeared. Meanwhile, the C
O and 1636 cm−1 ascribed to amine groups (c) indicated the formation of urethane bonds between Fe3O4@SiO2 and estrone, suggesting that the template estrone was immobilized on the surface of Fe3O4@SiO2 particles. After deposited TiO2 film (d), the strong peaks at 1090 cm−1 disappeared. Meanwhile, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks at 1718 cm−1 disappeared, confirming the removal of template estrone and the presence of the molecularly imprinted sites.
O peaks at 1718 cm−1 disappeared, confirming the removal of template estrone and the presence of the molecularly imprinted sites.
 | ||
| Fig. 2 The FT-IR spectra of (a) Fe3O4; (b) Fe3O4@SiO2; (c) Fe3O4@SiO2–estrone and (d) Fe3O4@SiO2@imprinted TiO2. | ||
The magnetic hysteresis loops and the dispersion and agglomeration process of Fe3O4 nanoparticles and the imprinted Fe3O4@SiO2@TiO2 were illustrated in Fig. 3. The shape of magnetic hysteresis loop of MIPs was similar to the Fe3O4 nanoparticles while the saturation magnetization value was slightly lower than that of the latter. The MIPs homogeneously dispersed in water could go straight towards the magnet and adhere to the inner side wall of the vials when external magnetic field was applied, and the turbid solution became clear and transparent. The results showed that the obtained materials could be separated and collected effectively and simply.
 | ||
| Fig. 3 Magnetic hysteresis loops of Fe3O4 nanoparticles (a) and Fe3O4@SiO2@TiO2 (b), and the insets show the dispersion (upper) and agglomeration (below) process of the Fe3O4@SiO2@TiO2 nanoparticles. | ||
3.2. Binding properties of the MIPs
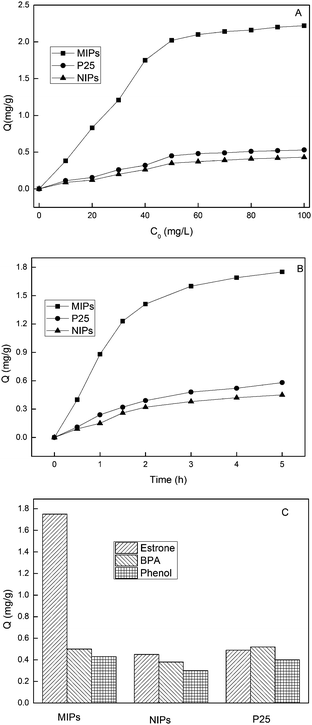
Binding capacity is an important index to evaluate the performances of MIPs. Binding properties including static adsorption equilibrium, binding kinetics and binding selectivity were evaluated by batch adsorption experiments. Static adsorption experiments were first performed in order to evaluate the adsorption capacities of MIPs at concentrations of 0–100 mg L−1 from aqueous solutions and the results were displayed in Fig. 4A. As seen, the amounts of estrone adsorbed per unit mass of MIPs increased with the increase of initial concentrations of estrone. At higher equilibrium concentration than 60 mg L−1, the adsorption capacity became stable. Also as observed, the adsorption capacity of MIPs was about 4 times of NIPs and P25. There were no specific recognition sites in NIPs and P25, so their binding capacities were relatively low. Moreover, it was found the binding capacity of P25 was slightly higher than that of NIPs, which might well because that the particle size of P25 (diameter 25 nm, surface area 53.5 m2 g−1) was smaller than NIPs (diameter 380 nm, surface area 46.7 m2 g−1).The Scatchard equation was employed to evaluate the maximum binding capacity of the MIPs, expressed as eqn (1):
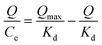 | (1) |
Fig. 4B shows the time dependent evolutions of estrone amount bound by MIPs, NIPs and P25. It was observed that the adsorption amounts of estrone onto the three materials increased with time. MIPs took up 50% of the equilibrium adsorption amount during 1 h and nearly reached saturation state within 4 h. And, more importantly, to attain the same adsorption capacity (the equilibrium adsorption capacity of NIPs or P25), MIP required only one eighth of time required by NIPs or P25. Hence, the MIPs had fast binding kinetics.
In order to investigate the competitive recognition ability of MIPs, two structural analogues of BPA and phenol were employed. As seen from Fig. 4C, the NIPs and P25 presented similar binding capacities for the three compounds. However, the binding capacity of MIPs for estrone was much higher than that for two analogues. The results showed that there were no selective recognition sites in NIPs and P25, while selective recognition sites existed in MIPs. MIPs could selectively bind estrone from other phenols, which opened a promising way to selective extraction and photodegradation of estrone.
3.3. Photodegradation of estrone
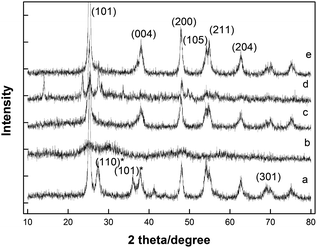
It is well known that the anatase TiO2 has higher photocatalytic degradation ability than rutile TiO2. So before carrying out the photodegradation experiments, the crystal forms of the deposited TiO2 were characterized by XRD, and the results were displayed in Fig. 5. As seen from Fig. 5a, the P25 TiO2 nanoparticles (anatase, hydrophilia) were the mixture of anatase and rutile. The dominant peaks at 2θ of 25.3°, 37.9°, 48.0°, 53.9°, 55.1° and 62.7° were corresponding to the (101), (004), (200), (105), (211), and (204) reflection of anatase TiO2, respectively, while the peaks at 2θ of 27.4°, 36.1° and 69.0° corresponding to the (110), (101) and (301) reflections of rutile phase. From Fig. 5b–e, we can see without calcination, the deposited TiO2 did not show anatase crystal form (cure b and d), so the step of calcination was necessary. Compared with TiO2 deposited in the absence of P25 (cure c), the TiO2 deposited in the presence of P25 (cure e) was more similar to anatase TiO2. Thus, in order to realize photodegradation, P25 was used as crystallization revulsant and calcination was carried out to complete conversion of the crystalline.The photodegradation experiments were carried out in single systems (one organic substrate in the suspension) and binary systems (coexisting two pollutants in the suspension). Fig. 6A shows the kinetic data for photodegradation of estrone over different photocatalysts in single systems. The kinetic data showed that all the degradation processes followed the first-order kinetics as eqn (2):
 | (2) |
Fig. 6B shows the kinetic data for the photodegradation of different phenols by MIPs in the single systems. The kapp value of BPA and phenol over MIPs were 0.021 and 0.017 min−1, being only 30.4% and 24.6% of that estrone over, respectively. The degradation of the target estrone was remarkably enhanced by the MIPs layer while the degradation of the non-target pollutant was correspondingly depressed by the MIPs coating. This enhanced photocatalytic selectivity can be attributed to molecule selective adsorption on the MIPs coatings. Notably, the ratio of ktarget to knon-target increased with the concentration decrease of the organic compounds. This indicated that the selectivity of MIPs was further improved when the initial concentration of the target pollutant was decreased, which would be especially in favor of selectively removing the low-level target pollutant in the presence of high-level non-target pollutants.
Binary system photocatalysis degradation experiments were also carried out to assess the selective photocatalysis degradation ability of MIPs. As seen from Table 1, the kapp values for the target's degradation over the MIPs were greater than those over P25, and the kapp values for the coexisted non-target over the MIPs were smaller than those over P25. And more importantly, R, the ratios of kapp values (ktarget/knontarget) over MIPs were much higher than those over P25. The results of binary system further confirmed that MIPs enhanced the photocatalytic selectivity towards the target contaminant. That MIPs enhanced the degradation of target estrone and depressed that of nontarget pollutants is very likely because a large number of selective recognition sites existed in the surface of MIPs. The high sensitivity would facilitate differentiating targeted estrone from coexistent compounds during their photocatalytic degradation and thereby realize highly selective photocatalytic degradation and removal of estrone.
| Coexisted compounds | Imprinted Fe3O4@SiO2@TiO2 (MIPs) | P25 | ||||
|---|---|---|---|---|---|---|
| ktarget (×103) | knon-target (×103) | R | ktarget (×103) | knon-target (×103) | R | |
| a R is the ratio of ktarget/knontarget. | ||||||
| Estrone | 85 ± 2 | — | 0.026 ± | — | ||
| 1× BPA | 67 ± 3 | 19 ± 4 | 3.5 | 15 ± 1 | 23 ± 2 | 0.65 |
| 1× Phenol | 73 ± 3 | 21 ± 3 | 3.5 | 17 ± 2 | 27 ± 2 | 0.63 |
| 10× BPA | 61 ± 2 | 11 ± 3 | 5.5 | 9 ± 2 | 19 ± 1 | 0.47 |
| 10× Phenol | 68 ± 4 | 15 ± 2 | 4.5 | 11 ± 1 | 21 ± 1 | 0.52 |
The MIPs displayed high selectivity in the photocatalytic removal of low-level target pollutants in the presence of high-level other pollutants. This can be explained as schematically shown in Scheme 1B. The whole photodegradation procedure includes two steps: first, the target compounds should be sufficiently adsorbed by TiO2, and then those adsorbed compounds were photodegradated. The adsorption of targeted compounds onto the photocatalyst might be the rate determining step, which could be deduced from Fig. 4B and 6 (the kinetics of estrone adsorption on MIPs (about 5 h) was lower than the photocatalytic degradation of estrone over Fe3O4@SiO2@TiO2 (about 1 h)). When the slowest step in the degradation process was enhanced, the total degradation process would be greatly accelerated. For the MIPs, many specific recognition sites existing in TiO2 layer facilitated the adsorption of target template molecules. So, the absorption rate of target molecules was higher than that of those non-target compounds, as well as the absorption rate of MIPs was higher than that of NIPs. The tendency was in good agreement with the photodegradation ability. The advantage of “preferential adsorption and preferential degradation” was more obvious when the concentration of target was low or the structure difference between target molecules and non-target molecules was large. Therefore, the enhanced photocatalyic selectivity can be attributed to molecularly selective adsorption on the MIPs coatings. The view agreed well with some reported works.7,16,24 Considering the difference between natural water and standard solutions studied above, photodegradation of estrone in real water samples, like tap water, underground water and river water were also studied. The water samples were collected in Nalgene bottles and filtered through 0.45 μm pore size membrane to remove the suspended particles before use. Initially, estrone was not detected in real water sample, so standard addition method was adopted to spike estrone into the real water samples. The results of photodegradation of estrone in real water samples in the presence and absence of non-target compounds were listed in Table 2. As seen, the kapp values in real water sample were all lower than that in standard solutions: kapp value in tap water was slightly lower than that in standard solution, while kapp value in river water was substantially decreased. This phenomenon can be ascribed to the fact that the tested tap water was purified water, which was more similar to the standard solution in components. Some metal ions, organic compounds and salts existed in river water, which were easy to capture the photo-generated electrons, reduce photoproduction holes or adsorb and scatter the radiation. As a result, the photolysis speed in river water was retarded. When 10 times of BPA or phenol was also spiked in those real water samples, kapp values displayed slight differences in standard solution and real water. This phenomenon may be explained as follows: the concentration of spiked BPA or phenol was far higher than the total amount of MIPs present in the real water, so the impact of material present in the real water on photocatalytic reaction could be ignored. Overall, the imprinted Fe3O4@SiO2@TiO2 (MIPs) displayed selective catalytic property in real water samples.
| Standard solution | Tap water | Ground water | River water | |||||
|---|---|---|---|---|---|---|---|---|
| ktarget (×103) | knon-target (×103) | ktarget (×103) | knon-target (×103) | ktarget (×103) | knon-target (×103) | ktarget (×103) | knon-target (×103) | |
| Estrone | 85 ± 2 | — | 82 ± 2 | — | 79 ± 2 | — | 68 ± 3 | — |
| 10× BPA | 61 ± 2 | 11 ± 3 | 61 ± 3 | 11 ± 4 | 60 ± 2 | 10 ± 2 | 57 ± 4 | 9 ± 3 |
| 10× phenol | 68 ± 4 | 15 ± 2 | 68 ± 3 | 14 ± 3 | 67 ± 3 | 14 ± 2 | 63 ± 3 | 13 ± 2 |
3.4. Reusability of the MIPs
The recovery and reusability of the photocatalyst are important in the photodegradation of organic pollutants, which is likely to be a key factor in improving the economic efficiency. To evaluate the lifetime of imprinted Fe3O4@SiO2@TiO2, the photodegradation of estrone (10 mg L−1) was carried out in successive batches. During the experiments, the MIPs were simply recovered by external magnet. In the first cycle, the degradation was conducted by irradiating for 1 h. After separated and rinsed with distilled water to remove the residual estrone and byproducts, the MIPs were poured into the refreshed pollutant solution and then the irradiation was continued again for 1 h as the second cycle. This process was repeated and the kinetic data for parts of the degradation cycles are shown in Fig. 7. The results showed that the prepared MIPs could be used repeatedly at least 10 times without significant decrease in their kapp values. This indicated that the MIPs had excellent photochemical stability during the photodegradation process. | ||
| Fig. 7 Kinetic data for the repeated photocatalytic degradation of estrone over imprinted Fe3O4@SiO2@TiO2. The numbers were cycle number. | ||
3.5. Comparison with other methods
Many excellent works about photodegradation of pollutants based on TiO2 nanoparticles have been reported. Many strategies have been proposed to improve the photocatalytic activity of TiO2 (ref. 25–29); for instance, MIPs based TiO2 nanocomposites have been prepared in order to improve the selectivity of TiO2 particles. Several typical examples about MIPs-TiO2 were summarized in Table 3.8–10,16,30–32 As can be seen from the table, selective removal of organic pollutants using directly imprinted TiO2 matrices is a hopeful method. The Fe3O4@imprinted TiO2 nanoparticles developed in this work exhibit high adsorption capacity, selectivity, and molecular recognitive photocatalytic activity for the target contaminant. Furthermore, it can provide fast separation ability, which is important for reuse of photocatalyst. So, the Fe3O4@imprinted TiO2 nanoparticles are ideal candidates for photodegradation of pollutants.| Photocatalysts | Target pollutant | Preparation method | Characteristic | Ref. |
|---|---|---|---|---|
| TiO2@organic MIP | Chlorophenols | In situ polymerization | (1) Higher selectivity; (2) lower stability; (3) multistep procedure | 8 |
| Nitrophenols | In situ polymerization | 9 | ||
| TiO2@inorganic MIP | Diethyl phthalate | Al3+ doping sol-gel method | (1) Higher stability; (2) mild polymerization conditions; (3) weak interaction between template and recognition sites | 10 |
| Dichlorophenol | Cu2+ doping sol-gel method | 30 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Matrices @imprinted TiO2 | ||||
| TiO2/WO3 | 4-Nitrophenol | One step sol-gel process | (1) Facile one-step sol-gel process | 31 |
| 2-Nitrophenol | ||||
| Glass | Salicylic acid | Liquid phase deposition | (1) Easy of template removal | 16 |
| TiO2 NT | Anthracene-9-carboxylic acid | Surface sol-gel process | (1) Enhanced photocatalytic activity; (2) photostability | 32 |
| Fe3O4 | Estrone | Liquid phase deposition | (1) Fast magnetic separation; (2) higher photocatalyst ability; (3) easy of template removal | This work |
4. Conclusions
In conclusion, estrone imprinted TiO2 hybridized film was prepared by LPD method on the surface of magnetic Fe3O4 particles. The imprinted Fe3O4@SiO2@TiO2 could achieve selective adsorption and photodegradation of the target estrone over other organic pollutants. By simply changing template molecules, novel Fe3O4 surface imprinted TiO2 can be prepared for other pollutants. Because of the high selectivity and excellent photocatalytic ability, the Fe3O4@imprinted TiO2 has a promising prospect in the treatment of wastewater. In addition to the selectivity, another challenge problem for TiO2 photocatalysis is the low utilization of visible light. The best source of light for photocatalyst is sunlight, which contains only a small part of ultraviolet light thereby improving the photocatalytic efficiency. Therefore, in future work, doping technique will be introduced to the process of depositing imprinted TiO2 film. We anticipate to achieving selective photodegradation of the target pollutant by sunlight. Moreover, multiple contaminants always coexist in real water body, so photocatalytic process for multi-targets should be further investigated.Acknowledgements
Financial support from the National Natural Science Foundation of China (21307052, 21275158), the Natural Science Foundation of Shandong Province of China (ZR2013BL006), and the Scholarship Award for Excellent Doctoral Student granted by the Ministry of Education of China, is gratefully acknowledged.Notes and references
- D. P. Grover, J. L. Zhou, P. E. Frickers and J. W. Readman, J. Hazard. Mater., 2011, 185, 1005–1011 CrossRef CAS PubMed.
- Z. Lin, Q. He, L. Wang, X. Wang, Q. Dong and C. Huang, J. Hazard. Mater., 2013, 252–253, 57–63 CrossRef CAS PubMed.
- M. C. Burleigh, M. A. Markowitz, M. S. Spector and B. P. Gaber, Environ. Sci. Technol., 2002, 36, 2515–2518 CrossRef CAS.
- S. Yi and W. Zhuang, Environ. Sci. Technol., 2006, 40, 2396–2401 CrossRef CAS.
- B. Sun, E. P. Reddy and P. G. Smirniotis, Environ. Sci. Technol., 2005, 39, 6251–6259 CrossRef CAS.
- D. Robert, A. Piscopo and J. V. Weber, Sol. Energy, 2004, 77, 553–558 CrossRef CAS PubMed.
- O. V. Makarova, T. Rajh and M. C. Thurnaure, Environ. Sci. Technol., 2000, 34, 4797–4803 CrossRef CAS.
- X. Shen, L. Zhu, J. Li and H. Tang, Chem. Commun., 2007, 1163–1165 RSC.
- X. Shen, L. Zhu, G. Liu, H. Yu and H. Tang, Environ. Sci. Technol., 2008, 42, 1687–1692 CrossRef CAS.
- X. Shen, L. Zhu, C. Huang, H. Tang, Z. Yu and F. Deng, J. Mater. Chem., 2009, 19, 4843–4851 RSC.
- S. F. Xu, H. Lu, X. Zheng and L. X. Chen, J. Mater. Chem. C, 2013, 1, 4406–4422 RSC.
- L. X. Chen, S. F. Xu and J. H. Li, Chem. Soc. Rev., 2011, 40, 2922–2942 RSC.
- S. F. Xu, H. Lu, J. H. Li, X. Song, A. Wang, L. X. Chen and S. Han, ACS Appl. Mater. Interfaces, 2013, 5, 8146–8154 CAS.
- S. F. Xu, L. X. Chen, J. H. Li, Y. Guan and H. Lu, J. Hazard. Mater., 2012, 237–238, 347–354 CrossRef CAS PubMed.
- S. F. Xu, L. X. Chen, J. H. Li, W. Qin and J. Ma, J. Mater. Chem., 2011, 21, 12047–12053 RSC.
- X. Shen, L. Zhu, H. Yu, H. Tang and S. Liu, New J. Chem., 2009, 33, 1673–1679 RSC.
- J. Inoue, T. Ooy and T. Takeuchi, Soft Matter, 2011, 7, 9681–9684 RSC.
- Y. Li, Y. Li, L. Huang, Q. Bin, Z. Lin, H. Yang, Z. Cai and G. Chen, J. Mater. Chem. B, 2013, 1, 1256–1262 RSC.
- M. Tatemichi, M. Sakamoto, M. Mizuhata, S. Deki and T. Takeuchi, J. Am. Chem. Soc., 2007, 129, 10906–10910 CrossRef CAS PubMed.
- H. Deng, X. Li, Q. Peng, X. Wang, J. Chen and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 2782–2785 CrossRef CAS PubMed.
- J. Ge, Q. Zhang, T. Zhang and Y. Yin, Angew. Chem., Int. Ed., 2008, 47, 8924–8928 CrossRef CAS PubMed.
- X. Wang, L. Wang, X. He, Y. Zhang and L. Chen, Talanta, 2009, 78, 327–332 CrossRef CAS PubMed.
- C. D. Ki, C. Oh, S. Oh and J. Chang, J. Am. Chem. Soc., 2002, 124, 14838–14839 CrossRef CAS PubMed.
- X. Shen, L. Zhu, G. Liu, H. Tang, S. Liu and W. Li, New J. Chem., 2009, 33, 2278–2285 RSC.
- G. Jiang, Z. Lin, L. Zhu, Y. Ding and H. Tang, Carbon, 2010, 48, 3369–3375 CrossRef CAS PubMed.
- G. Jiang, Z. Lin, C. Chen, L. Zhu, Q. Chang, N. Wang, W. Wei and H. Tang, Carbon, 2011, 49, 2693–2701 CrossRef CAS PubMed.
- Y. Chi, Q. Yuan, Y. Li, L. Zhao, N. Li, X. Li and W. Yan, J. Hazard. Mater., 2013, 262, 404–411 CrossRef CAS PubMed.
- C. Zhan, F. Chen, J. Yang, D. Dai, X. Cao and M. Zhong, J. Hazard. Mater., 2014, 267, 88–97 CrossRef CAS PubMed.
- L. Gu, J. Wang, Z. Zou and X. Han, J. Hazard. Mater., 2014, 268, 216–223 CrossRef CAS PubMed.
- D. M. Han, G. L. Dai, W. P. Jia and H. D. Liang, Micro Nano Lett., 2010, 5, 76–80 CAS.
- X. Luo, F. Deng, L. Min, S. Luo, B. Guo, G. Zeng and C. Au, Environ. Sci. Technol., 2013, 47, 7404–7412 CAS.
- Y. Liu, R. Liu, C. Liu, S. Luo, L. Yang, F. Sui, Y. Teng, R. Yang and Q. Cai, J. Hazard. Mater., 2010, 182, 912–918 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |