β-BaGa[B4O8(OH)](H2O) and Ba4Ga[B10O18(OH)5](H2O): new barium galloborates featuring unusual [B4O8(OH)]5− and [B10O18(OH)5]11− clusters†
Hui Yangab,
Chun-Li Hua,
Jun-Ling Songa and
Jiang-Gao Mao*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: mjg@fjirsm.ac.cn; Fax: +86-591-83714946; Tel: +86-591-83704836
bUniversity of the Chinese Academy of Sciences, Beijing 100039, P. R. China
First published on 11th September 2014
Abstract
Two new barium galloborates, namely, β-BaGa[B4O8(OH)](H2O) (1) and Ba4Ga[B10O18(OH)5](H2O) (2), have been synthesized by hydrothermal reactions. Compounds 1 crystallizes in centrosymmetric space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and displays two-dimensional (2D) anionic layers composed of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers interconnected by B–O–Ga linkages, furthermore Ba2+ ions and water molecules are located at the interlayer space. The neighbouring galloborate layers are connected via hydrogen bonds of water molecules. Compound 2 crystallizes in a polar space group Cc. In the structure, [B10O18(OH)5]11− clusters and [GaO4]5− tetrahedra are connected with each other to form a 3D network filled by Ba2+ ions and water molecules which consist of 1D tunnels based on unusual Ga3B16 19-member rings (MRs). The water molecules are coordinated with the Ba2+ ions and also form hydrogen bonds with the galloborate network. Second harmonic generation (SHG) measurements indicate that compound 2 displays a weak SHG response of about 0.2 times that of KH2PO4 (KDP). Optical properties, ferroelectric properties, piezoelectric properties, thermal stability and theoretical calculations based on density functional theory (DFT) methods of both compounds have also been studied.
and displays two-dimensional (2D) anionic layers composed of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers interconnected by B–O–Ga linkages, furthermore Ba2+ ions and water molecules are located at the interlayer space. The neighbouring galloborate layers are connected via hydrogen bonds of water molecules. Compound 2 crystallizes in a polar space group Cc. In the structure, [B10O18(OH)5]11− clusters and [GaO4]5− tetrahedra are connected with each other to form a 3D network filled by Ba2+ ions and water molecules which consist of 1D tunnels based on unusual Ga3B16 19-member rings (MRs). The water molecules are coordinated with the Ba2+ ions and also form hydrogen bonds with the galloborate network. Second harmonic generation (SHG) measurements indicate that compound 2 displays a weak SHG response of about 0.2 times that of KH2PO4 (KDP). Optical properties, ferroelectric properties, piezoelectric properties, thermal stability and theoretical calculations based on density functional theory (DFT) methods of both compounds have also been studied.
Introduction
During the last few decades, second-order nonlinear optical (NLO) materials have attracted considerable attention because of their great practical application in photonic technologies.1 Among them, borates have attracted a great deal of research attention due to their rich structural chemistry, high damage threshold, and large nonlinear optical efficiency.2 Many non-centrosymmetric borate crystals have been reported including β-BaB2O4 (BBO), LiB3O5 (LBO), and KBeBO3F2 (KBBF).3 B atom can adopt two different basic coordination geometries: planar triangle with π-conjugated system (BO3) and tetrahedron (BO4). Furthermore, these BO3 and BO4 groups can be polymerized into a wide variety of anionic structures such as 1D chains, 2D sheets, or 3D networks in addition to isolated clusters.4 Introduction of the heteroatom into the borate system has been proved to be an effective route for the preparations of new borates with novel topologies and enhanced second harmonic generation (SHG) properties. Recently, the family of metal borates have been expanded greatly to synthesize new classes of compounds such as borogermanates,5 borophosphates,6 aluminoborates,7 borosulfates,8 and boroberyllates.9Coupled with the fact that Ga3+ ion can also form various coordination geometries such as GaO4 tetrahedron, GaO5 trigonal bipyramid and GaO6 octahedron, we anticipate that the introduction of GaOn (n = 4, 5, 6) groups into the borate systems can also result in a large number of galloborates with novel structures and excellent NLO properties. So far, a number of the alkali and alkaline-earth gallium borates have been studied.10–20 The structurally characterized alkali metal galloborates include LiGa(OH)(BO3)(H2O),10 A2Ga(B5O10)(H2O)4 (A = Rb, K),10,11 K2Ga2O(BO3)2,12 A2Ga2O(BO3)2 (A = Na, K, Rb and Cs),13 and Li6Ga2B4O12,14 among which Rb2Ga(B5O10)(H2O)4 displays moderate strong SHG response of 1.0 × KDP (KH2PO4). A variety of alkaline earth metal galloborates have been also reported including MgGaBO4,15 CaGaBO4,16 two forms of SrGaBO4,16,17 AeGa2B2O7 (Ae = Sr, Ba),18 BaGa[B4O8(OH)](H2O)19 and Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] which displays moderately strong SHG response of 3.0 × KDP.20 The structure of BaGa2B2O7 consists of a framework structure of corner-sharing tetrahedral (GaO4) chains and pyroborate (B2O5) groups with interspaces occupied by the eight-coordinated Ba2+ cations.18 The structure of BaGa[B4O8(OH)](H2O) features a layered anionic framework composed of [B5O9(OH)]-like [GaB4O11(OH)] clusters that are interconnected by Ga–O–Ga likages.19 The structure of Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] exhibits a 3D network with 14-member ring (MR) channels along the [100], [101], and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01] directions based on GaO4 tetrahedra, B3O6(OH) and B4O7(OH)2 clusters.20
01] directions based on GaO4 tetrahedra, B3O6(OH) and B4O7(OH)2 clusters.20
The chemical compositions and structures of metal borates isolated are very sensitive to synthetic conditions such as temperature, the size and charge of cations, metal–borate ratio, pH value of the reaction media, etc. For example, K2[Ge(B4O9)]2·H2O, K4[B8Ge2O17(OH)2] and KBGe2O6 were isolated from the same system under different synthetic conditions.21 With K2[B4O5(OH)]·2H2O as a boron source and a mixture of water, pyridine and diethylenetriamine as solvent, K2[Ge(B4O9)]2·H2O was obtained at 170 °C, whereas K4[B8Ge2O17(OH)2)] was prepared from a flux of K2[B4O5(OH)]·2H2O at 280 °C. KBGe2O6 was prepared by using K2B4O7·4H2O as a boron source in a mixture of water, ethylene glycol and 1,4-diazabicyclo[2,2,2]octane at 170 °C. We expect that the galloborate system will also be strongly affected by subtile changes of reaction conditions. Therefore, in order to further understand the relationship between the structures of the products formed and the reaction conditions, we started a research program to explore barium gallium borates systematically. Our research efforts led to the isolation of two new members in the Ba–Ga–B–O family, namely, β-BaGa[B4O8(OH)](H2O) (1) and Ba4Ga[B10O18(OH)5](H2O) (2). Herein, we report their syntheses, crystal structures, ferroelectric properties as well as optical properties.
Experimental section
Materials and methods
H3BO3 (Shanghai Reagent Factory, 99.99%), Ga2O3 (Shanghai Reagent Factory, 99.99%), and Ba(OH)2·8H2O (Alfa Aesar, 99.0%), Na2B4O7·10H2O (Shanghai Reagent Factory, 99.99%), BaSO4 (Alfa Aesar, 99.0%) were used without further purification. IR spectra were recorded on a Magna 750 FT-IR spectrometer as KBr pellets in the range of 4000–400 cm−1. Microprobe elemental analyses were performed on a field-emission scanning electron microscope (JSM6700F) equipped with an energy-dispersive X-ray spectroscope (Oxford INCA). X-Ray powder diffraction (XRD) patterns were collected on a XPERT-MPD θ–2θ diffractometer using graphite-monochromated Cu-Kα radiation with a step size of 0.02°. Optical diffuse-reflectance spectra were measured at room temperature with a PE Lambda 900 UV-visible spectrophotometer. The BaSO4 plate was used as a standard (100% reflectance). The absorption spectrum was calculated from reflectance spectrum using the Kubelka–Munk function: α/S = (1R)2/2R,22 where α is the absorption coefficient, S is the scattering coefficient, which is practically wavelength-independent when the particle size is larger than 5 μm, and R is the reflectance. Thermogravimetric analyses (TGA) were carried out with a NETZSCH STA 449C unit at a heating rate of 10 °C min−1 under nitrogen atmosphere and differential scanning calorimetry (DSC) analyses were performed on a NETZSCH DTA404PC unit at heating rate of 10 °C min−1 under nitrogen atmosphere. Measurements of the powder frequency-doubling effect were carried out by means of the modified method of Kurtz and Perry.23 A 1064 nm radiation generated by a Q-switched Nd:YAG solid-state laser was used as the fundamental frequency light. The SHG wavelength is 532 nm. Thus, the sample was ground and sieved into a specific particle size range (100–150 μm). Sieved KDP powder (100–150 μm) was used as a reference material to assume the SHG effect. The ferroelectric property for compound 2 was measured on an aixACCT TF Analyzer 2000E ferroelectric tester at room temperature and piezoelectric coefficient was measured by using a quasistatic d33 meter (Institute of Acoustics, Chinese Academy of Sciences, model ZJ-4AN). The powder was pressed into a pellet (5 mm diameter and 0.4 mm thick), and the conducting Ag-glue was applied on the both sides of the pellet surfaces for electrodes.Synthesis of β-BaGa[B4O8(OH)](H2O) (1)
A mixture of BaSO4 (0.0812 g, 0.35 mmol), Ga2O3 (0.0428 g, 0.22 mmol), and H3BO3 (0.1240 g, 2 mmol) in 3.0 mL H2O with the pH value of 6.0 was sealed in an autoclave equipped with a Teflon liner (23 mL) and heated first at 100 °C for 5 h, and then 220 °C for 4 days followed by slow cooling to room temperature at a rate of 2.3 °C h−1. The final pH value was close to 6.0. Colorless block crystals of compound 1 were collected in about 70% yield based on Ga. Its purity was confirmed by XRD powder diffraction study (Fig. S1a in the ESI†). The average molar ratio of Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga in compound 1 determined by energy-dispersive spectrometry (EDS) on several single crystals is 1.2
Ga in compound 1 determined by energy-dispersive spectrometry (EDS) on several single crystals is 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is in good agreement with that determined from single-crystal X-ray structural studies. It is unsuccessful to synthesize compound 1 with other reactants like Ba(OH)2·8H2O and BaCl2·2H2O. IR data (KBr cm−1): 3441 (m), 1679 (m), 1410 (s), 1222 (s), 1034 (s), 921 (s), 853 (w), 740 (w), 658 (w).
1, which is in good agreement with that determined from single-crystal X-ray structural studies. It is unsuccessful to synthesize compound 1 with other reactants like Ba(OH)2·8H2O and BaCl2·2H2O. IR data (KBr cm−1): 3441 (m), 1679 (m), 1410 (s), 1222 (s), 1034 (s), 921 (s), 853 (w), 740 (w), 658 (w).
Synthesis of Ba4Ga[B10O18(OH)5](H2O) (2)
A mixture of Ba(OH)2·8H2O (0.3150 g, 1 mmol), Ga2O3 (0.0930 g, 0.5 mmol), H3BO3 (0.1240 g, 2 mmol) in 3.0 mL H2O with the pH value of 14.0 was sealed in an autoclave equipped with a Teflon liner (23 mL) and heated at 100 °C for 5 h, then heated at 220 °C for 4 days followed by slow cooling to room temperature at a rate of 2.3 °C h−1 with the final pH value of 9.0. The product was washed with hot water and then dried in air. Colorless brick crystals of compound 2 were collected in about 30% yield based on Ga after sieving by ultrasound. Its purity was confirmed by XRD power diffraction studies (Fig. S1b in the ESI†). The average molar ratio of Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga in compound 2 determined by energy-dispersive spectrometry (EDS) on several single crystals is 3.8
Ga in compound 2 determined by energy-dispersive spectrometry (EDS) on several single crystals is 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is in good agreement with that determined from single-crystal X-ray structural analyses. IR data (KBr cm−1): 3405 (w), 1624 (w), 1341 (s), 1244 (w), 856 (m), 774 (w), 556 (w).
1, which is in good agreement with that determined from single-crystal X-ray structural analyses. IR data (KBr cm−1): 3405 (w), 1624 (w), 1341 (s), 1244 (w), 856 (m), 774 (w), 556 (w).
Single-crystal structure determination
Data collections for both compounds were performed on SuperNova (Mo) X-ray Source, Mo-Kα radiation (λ = 0.71073 Å) at 293(2) K. Both data sets were corrected for Lorentz and polarization factors as well as for absorption by the multi-scan method.24a Both structures were solved by direct methods and refined by a full-matrix least-squares fitting on F2 by SHELX-97.24b All hydrogen atoms are located at geometrically calculated positions and refined with isotropic thermal parameters. The refined Flack factor of −0.02(7) for compound 2 is close to zero, confirming the correctness of its absolute structure. Both structures were also checked for possible missing symmetry with the program PLATON.24c Crystallographic data and structural refinements for the two compounds are summarized in Table 1 and important bond distances are listed in Table S1.† More information about the crystallographic studies as well as atomic displacement parameters are given as ESI.†| Formula | β-BaGa(B4O8(OH))(H2O) (1) | Ba4Ga(B10O18(OH)5)(H2O) (2) |
|---|---|---|
| a R1 = ∑||Fo| − |Fc||/∑|Fo|, wR2 = {∑w[(Fo)2 − (Fc)2]2/∑w[(Fo)2]2}1/2. | ||
| Fw | 413.32 | 1118.24 |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Cc |
| a [Å] | 7.0811(6) | 7.0097(3) |
| b [Å] | 7.1144(7) | 12.2089(5) |
| c [Å] | 9.8431(8) | 22.5507(9) |
| α [°] | 106.946(8) | 90 |
| β [°] | 91.245(7) | 96.798(4) |
| γ [°] | 119.145(9) | 90 |
| V [Å3] | 406.26(6) | 1916.34(14) |
| Z | 2 | 4 |
| Dcalcd [g cm−3] | 3.379 | 3.876 |
| μ [mm−1] | 8.174 | 9.612 |
| F(000) | 380 | 2016 |
| GOF on F2 | 1.111 | 1.017 |
| R1, wR2 (I > 2σ(I))a | 0.0386, 0.0922 | 0.0212, 0.0414 |
| R1, wR2 (all data) | 0.0425, 0.0982 | 0.0221, 0.0418 |
Computational descriptions
Single crystal structural data of both compounds were used for the theoretical calculations. Band structures and density of states (DOS), and optical properties were performed with the total energy code CASTEP.25 The total energy is calculated with density functional theory (DFT) using the Perdew–Burke–Ernzerhof in the generalized gradient approximation.26 The interactions between the ionic cores and the electrons are described by the norm-conserving pseudopotential.27 The following orbital electrons are treated as valence electrons: Ba-5s25p66s2, Ga-3d104s24p1, B-2s22p1, O-2s22p4 and H-1s1. The number of plane waves included in the basis set is determined by a cut off energy of 800 eV. In addition, the numerical integration of the Brillouin zone is performed using a 4 × 4 × 3 and 4 × 2 × 1 Monkhorst–Pack k-point sampling for compounds 1 and 2, respectively. The other calculating parameters and convergent criteria were the default values of the CASTEP code.Result and discussion
Two new barium galloborates, namely, β-BaGa[B4O8(OH)](H2O) (1) and Ba4Ga[B10O18(OH)5](H2O) (2), were prepared by hydrothermal reactions. It is interesting to note that two compounds were synthesized under the same reaction temperature (220 °C) but different starting materials and molar ratios. β-BaGa[B4O8(OH)](H2O) (1) was prepared from a mixture of BaSO4, Ga2O3, and H3BO3 with molar ratio of 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, whereas Ba4Ga[B10O18(OH)5](H2O) (2) was obtained from a mixture of Ba(OH)2·8H2O, Ga2O3, and H3BO3 in a molar ratio of 2
10, whereas Ba4Ga[B10O18(OH)5](H2O) (2) was obtained from a mixture of Ba(OH)2·8H2O, Ga2O3, and H3BO3 in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. It is interesting to note that the previously reported α-BaGa[B4O8(OH)](H2O) phase was prepared from a mixture of barium hydroxide, gallium isopropoxide, and boric acid (Ba
4. It is interesting to note that the previously reported α-BaGa[B4O8(OH)](H2O) phase was prepared from a mixture of barium hydroxide, gallium isopropoxide, and boric acid (Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B molar ratio = 1.5
B molar ratio = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) in a mixed solvent of water and pyridine at 260 °C.19 Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] was isolated by hydrothermal reaction of a mixture of H3BO3, Ga(iPrO)3 and Ba(OH)2 (molar ratio = 4
10) in a mixed solvent of water and pyridine at 260 °C.19 Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] was isolated by hydrothermal reaction of a mixture of H3BO3, Ga(iPrO)3 and Ba(OH)2 (molar ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in water at 220 °C for 10 days.20 Hence the barium galloborates isolated are very sensitive to the gallium source, Ba/Ga/B molar ratio, reaction temperature and reaction media used. The structures of centrosymmetric β-BaGa[B4O8(OH)](H2O) (1) and polar Ba4Ga[B10O18(OH)5](H2O) (2) feature two different types of anionic open frameworks based on two types of borate clusters ([B4O8(OH)]5− and [B10O18(OH)5]11−) which are further interconnected by [GaO4]5− or dimeric [Ga2O8]10− units, respectively. The polar compound 2 displays weak second-harmonic generation response of about 0.2 times that of KH2PO4 (KDP).
2) in water at 220 °C for 10 days.20 Hence the barium galloborates isolated are very sensitive to the gallium source, Ba/Ga/B molar ratio, reaction temperature and reaction media used. The structures of centrosymmetric β-BaGa[B4O8(OH)](H2O) (1) and polar Ba4Ga[B10O18(OH)5](H2O) (2) feature two different types of anionic open frameworks based on two types of borate clusters ([B4O8(OH)]5− and [B10O18(OH)5]11−) which are further interconnected by [GaO4]5− or dimeric [Ga2O8]10− units, respectively. The polar compound 2 displays weak second-harmonic generation response of about 0.2 times that of KH2PO4 (KDP).
Structural description
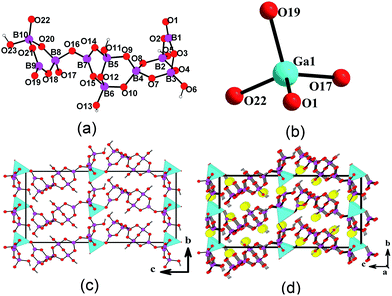
β-BaGa[B4O8(OH)](H2O) (1) crystallizes in the centrosymmetric space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Its structure features a layered anionic framework composed of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers with Ba2+ ions and water molecules located at the interlayer space (Fig. 1d), which is similar to that of α-BaGa[B4O8(OH)](H2O) which crystallizes in monoclinic space group C2/c.19 The asymmetric unit of 1 contains one barium, one gallium, one [B4O8(OH)]5− cluster and a water molecule. The Ga3+ ion is five coordinated with a trigonal–bipyramidal coordination geometry. A pair of GaO5 units forms a [Ga2O8]10− dimer via edge-sharing (O(8)–O(8)) (Fig. 1b). The Ga–O distances range from 1.855(4) to 2.055(5) Å and O–Ga–O bond angles fall in the range from 77.7(2) and 170.48(1)°. Within the [B4O8(OH)]5− cluster, B(1), B(3) and B(4) atoms are three coordinated in a planar trigonal geometry whereas B(2) is tetrahedral coordinated. The B–O distances are in the range of 1.339(9) to 1.411(8) Å and 1.453(9) to 1.483(8) Å, and O–B–O bond angles range from 114.2(6) to 124.6(6)° and 105.3(5) to 111.7(5)° for BO3 and BO4 groups, respectively. These bond lengths and angles are comparable to those previously reported in α-BaGa[B4O8(OH)](H2O) and other related galloborates.18–20 B(2)O4, B(3)O3 and B(4)O3 form a common [B3O7]5− cluster via corner-sharing. The B(1)O3 group is attached to the [B3O7]5− cluster by B(2)–O(3)–B(1) linkage, forming a [B4O8(OH)]5− anion (Fig. 1a). The interconnection of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers via B–O–Ga linkages result in a [GaB4O8(OH)]2− layer parallel to the ab plane (Fig. 1c). The interlayer distance is about 9.84 Å. Ba2+ ions and water molecules are located at the interlayer space. The Ba2+ cation is ten coordinated by nine oxygen atoms from two neighboring galloborate layers as well as a water molecule with Ba–O distances ranging from 2.699(6) to 3.063(5) Å (Fig. S2 in the ESI†). The calculated total bond valances for Ba(1), Ga(1), B(1)–B(4) atoms are 2.18, 3.06, 3.06, 3.07, 2.98, 3.04, respectively, indicating that Ba, Ga and B atoms are in oxidation states of +2, +3 and +3, respectively.28 There are hydrogen bonds among water molecule, hydroxyl group and oxygen atoms of the borate cluster (O(1W)–H(1WA)⋯O(3) 2.696 Å; O(1)–H(8A)⋯O(2) 2.700 Å) (Table S2 in the ESI†) which provide additional stability for the structure.
. Its structure features a layered anionic framework composed of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers with Ba2+ ions and water molecules located at the interlayer space (Fig. 1d), which is similar to that of α-BaGa[B4O8(OH)](H2O) which crystallizes in monoclinic space group C2/c.19 The asymmetric unit of 1 contains one barium, one gallium, one [B4O8(OH)]5− cluster and a water molecule. The Ga3+ ion is five coordinated with a trigonal–bipyramidal coordination geometry. A pair of GaO5 units forms a [Ga2O8]10− dimer via edge-sharing (O(8)–O(8)) (Fig. 1b). The Ga–O distances range from 1.855(4) to 2.055(5) Å and O–Ga–O bond angles fall in the range from 77.7(2) and 170.48(1)°. Within the [B4O8(OH)]5− cluster, B(1), B(3) and B(4) atoms are three coordinated in a planar trigonal geometry whereas B(2) is tetrahedral coordinated. The B–O distances are in the range of 1.339(9) to 1.411(8) Å and 1.453(9) to 1.483(8) Å, and O–B–O bond angles range from 114.2(6) to 124.6(6)° and 105.3(5) to 111.7(5)° for BO3 and BO4 groups, respectively. These bond lengths and angles are comparable to those previously reported in α-BaGa[B4O8(OH)](H2O) and other related galloborates.18–20 B(2)O4, B(3)O3 and B(4)O3 form a common [B3O7]5− cluster via corner-sharing. The B(1)O3 group is attached to the [B3O7]5− cluster by B(2)–O(3)–B(1) linkage, forming a [B4O8(OH)]5− anion (Fig. 1a). The interconnection of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers via B–O–Ga linkages result in a [GaB4O8(OH)]2− layer parallel to the ab plane (Fig. 1c). The interlayer distance is about 9.84 Å. Ba2+ ions and water molecules are located at the interlayer space. The Ba2+ cation is ten coordinated by nine oxygen atoms from two neighboring galloborate layers as well as a water molecule with Ba–O distances ranging from 2.699(6) to 3.063(5) Å (Fig. S2 in the ESI†). The calculated total bond valances for Ba(1), Ga(1), B(1)–B(4) atoms are 2.18, 3.06, 3.06, 3.07, 2.98, 3.04, respectively, indicating that Ba, Ga and B atoms are in oxidation states of +2, +3 and +3, respectively.28 There are hydrogen bonds among water molecule, hydroxyl group and oxygen atoms of the borate cluster (O(1W)–H(1WA)⋯O(3) 2.696 Å; O(1)–H(8A)⋯O(2) 2.700 Å) (Table S2 in the ESI†) which provide additional stability for the structure.
The overall structures of the α- and β-forms of BaGa[B4O8(OH)](H2O) are quite similar.19 However some differences do exist. Firstly, they belong to two different crystal systems and space groups; secondly, the 2D galloborate layers are packed in different fashions along the c-axis. The β- and α-phases contain one and two repeated galloborate layers within their unit cells, respectively, hence the length of the c axis for α-phase is almost doubled compared with that of the β-form.
The structure of compound 1 is also closely related with that of K4[Ge2B8O17(OH)2].21b In K4[B8Ge2O17(OH)2], the 2D borogermanate layer is based on [B4O8(OH)]5− unit and [Ge2O7]6− dimer formed by two GeO4 tetrahedra. However, [B4O8(OH)]5− unit in K4[B8Ge2O17(OH)2] has a different shape from that in compound 1. The BO3 group is no longer hanging on the B3O7 group but bridges with two B atoms of the B3O7 group, hence [B4O8(OH)]5− unit in K4[B8Ge2O17(OH)2] forms two orthogonal B3O8 groups.
Ba4Ga[B10O18(OH)5](H2O) (2) crystallizes in the polar space group Cc. Its structure consists of a unique 3D network composed of [B10O18(OH)5]11− clusters and [GaO4]5− tetrahedra that are interconnected via Ga–O–B linkages, forming large 1D tunnels of Ga3B16 rings which are filled by Ba2+ ions and water molecules (Fig. 2d). The asymmetric unit of Ba4Ga[B10O18(OH)5](H2O) (2) consists of four Ba2+, one Ga3+, one [B10O18(OH)5]11− cluster and a water molecule. Within the [B10O18(OH)5]11− anion, B(1), B(7), and B(9) atoms form planar trigonal BO3 groups whereas the remaining B atoms are tetrahedrally coordinated. For the BO3 groups, the B–O distances are in the range of 1.356(7)–1.399(7) Å and O–B–O bond angles range from 113.8(5)–123.9(5)°. B–O distances and O–B–O angles for the BO4 tetrahedra are in the range of 1.429(6)–1.554(7) Å and 105.3(4)–115.7(5)°, respectively. The Ga3+ ion is tetrahedrally coordinated by four oxygen atoms from two [BO3] and two [BO4] groups from four different [B10O18(OH)5]11− clusters (Fig. 2b). The Ga–O bond distances and O–Ga–O bond angles are in the range of 1.806(4)–1.869(4) Å and 103.6(5)–112.5(8)°, respectively. These bond lengths and angles are comparable to those reported in α- and β-forms of BaGa[B4O8(OH)](H2O) and other galloborates previously reported.18–20
[B10O18(OH)5]11− cluster in Ba4Ga[B10O18(OH)5](H2O) is quite novel. It can be considered to be formed by a central B6O14 cluster corner-sharing with a B3O8 cluster and a B(1)O3 group in both ends (Fig. 2a). The central B6O14 cluster consists of three 3-MRs, the middle one is formed by three BO4 groups whereas the other two are formed by two BO4 and one BO3 groups. O(5), O(6), O(11), O(13) and O(23) atoms are protonated. To the best of our knowledge, such type of borate cluster has not been reported before.
The interconnection of [B10O18(OH)5]11− clusters and GaO4 tetrahedra via corner-sharing led to a novel 3D network (Fig. 2c). Each [B10O18(OH)5]11− polyanion connects with four GaO4 tetrahedra via corner-sharing (O(1), O(17), O(19) and (O22)) and each GaO4 also connects with four [B10O18(OH)5]11− clusters. Such connectivity resulted in the formation of a large 1D tunnels based on Ga3B16 19-MRs along the a axis. Each Ga3B16 ring consists of three GaO4, five BO3 and eleven BO4 groups. The tunnels are filled by Ba2+ cations and water molecules. Ba(1) and Ba(3) atoms are ten coordinated by ten oxide anions whereas Ba(2) and Ba(4) atoms are nine-coordinated by eight oxide anions and a water molecule (Fig. S3 in the ESI†). The Ba–O distances are in the range of 2.652(4)–3.180(4) Å. The calculated total bond valances for Ba1–Ba4, Ga1 are 2.04, 1.78, 2.40, 1.92, 3.02, respectively, and those for B1 to B10 are 3.01, 3.03, 3.03, 3.02, 3.02, 3.06, 3.06, 3.05, 3.05, 3.07, respectively, indicating that Ba, Ga and B are in oxidation states of +2, +3, and +3, respectively.28 The water molecule and hydroxyl groups of the borate cluster are involved in hydrogen bonding which provides additional stability for the network structure (Table S2 in the ESI†).
It is interesting to compare the structure of Ba4Ga[B10O18(OH)5](H2O) with that of Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] which contains two different types of borate clusters. The structure of Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] exhibits a 3D network structure with 14-MR channels along the [100], [101], and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01] directions based on GaO4 tetrahedra, B3O6(OH) and B4O7(OH)2 clusters.20
01] directions based on GaO4 tetrahedra, B3O6(OH) and B4O7(OH)2 clusters.20
Optical properties
β-BaGa[B4O8(OH)](H2O) (1) and Ba4Ga[B10O18(OH)5](H2O) (2) show strong absorption in the region of 200 to 370 nm and 200 to 418 nm, respectively (Fig. S4 in the ESI†). Both compounds show little absorption in the range of 420–2000 nm. Optical diffuse reflectance spectra reveal that compounds 1 and 2 are wide band gap semiconductors with optical band gaps around 4.65 and 4.12 eV, respectively (Fig. S5 in the ESI†). Both compounds display broad IR absorption bands at 3615, 3439 and 3264 cm−1 due to the presence of OH groups and H2O molecules. For compound 1, the absorption band at 1679 cm−1 is also assigned to the asymmetric stretching vibrations and symmetric bond-bending vibrations of O–H bonds. The vibration absorption bands at 1410–1220 cm−1 are due to B–O bond asymmetric stretching of the BO3 units, whereas those of BO4 units appeared at 1085–921 cm−1. The peaks at 658 and 740 cm−1 are due to the stretching vibration of GaO4 (Fig. S6a in the ESI†). Similar to compound 2, the absorption bands at 1240–1350 and 856 cm−1 can be assigned to the asymmetric stretch vibrations of the BO3 groups. The absorption peak at 1033 cm−1 can be assigned to the asymmetric stretch vibrations of the BO4 group. The absorption peak at around 961 cm−1 are due to the symmetric stretch vibrations of the BO3 group. The absorption bands at 770–808 cm−1 can be assigned to the symmetric stretch vibrations of BO4 group (Fig. S6b in the ESI†). The absorption bands with frequency below 600 cm−1 are difficult to be assigned undoubtedly due to the overlaps of the bending modes of BO4 and GaO4 polyhedron in the low frequency vibrations. These assignments are in agreement with those reported in other barium galloborates.18–20TGA and DSC studies
Thermogravimetric analysis (TGA) studies indicate that β-BaGa[B4O8(OH)](H2O) (1) shows a weight loss in the range of 300–650 °C under nitrogen atmosphere, which corresponds to the removal of 1.5 mol of water molecules per formula unit, and one endothermic peak at around 423 °C can be found in the DSC curve. The observed weight loss of 6.55% matches well with the calculated one (6.53%) (Fig. S7a in the ESI†). The endothermic peak at 817 °C corresponds to the melting of the dehydrated product. Ba4Ga[B10O18(OH)5](H2O) (2) displays one step of weight loss in the range of 480–600 °C, which corresponds to release of 3.5 mol of H2O molecules per formula unit. The observed weight loss of 5.63% is in agreement with the calculated one (6.03%) (Fig. S7b in the ESI†). This assignment is also in agreement with the endothermic peak at 504 °C in the DSC curve. The endothermic peak at 790 °C corresponds to the melting of the dehydrated product. Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] and α-BaGa[B4O8(OH)](H2O) can keep stable under 400 °C and 350 °C, respectively. Because α/β-BaGa[B4O8(OH)](H2O) have similar structures, their stability are simialar. And there exist more hydrogen bonds in Ba3Ga2[B3O6(OH)]2[B4O7(OH)2] and compound 2 comparing with that in α/β-BaGa[B4O8(OH)](H2O). So their stability is better than α/β-BaGa[B4O8(OH)](H2O). The residues obtained after thermal annealing (at 700 °C for 5 h) of two compounds are characterized by powder X-ray diffraction patterns which shows they may be new phases. (Fig. S1c and d in the ESI†). It may be reported in later work.SHG properties
Since Ba4Ga[B10O18(OH)5](H2O) (2) crystallizes in the polar space group Cc, it is worthy to study their SHG properties. SHG measurements on a 1064 nm Q-switched Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) YAG laser with the sieved crystal samples (100–150 μm) reveal that it displays weak SHG responses of 0.2 times that of KDP (Fig. 3).
YAG laser with the sieved crystal samples (100–150 μm) reveal that it displays weak SHG responses of 0.2 times that of KDP (Fig. 3).
 | ||
| Fig. 3 Oscilloscope traces of SHG signals for the powders (100–150 μm) of KDP and Ba4Ga[B10O18(OH)5](H2O) (2). | ||
According to the anionic-group theory, its SHG signal may mainly originate from the BO3 groups and small contribution from BO4 groups.29 Taking this approximation and neglecting the contribution from those Ba-coordinated polyhedra, the local dipole moments for the GaO4, BO3 and BO4 polyhedra and the net dipole moment within a unit cell were calculated by using a method reported earlier.30 The calculated dipole moments for the GaO4, BO4 and BO3 groups are 1.17 D, 0.84–2.14 D, and 0.98–1.46 D, respectively, which are in agreement with previously reported values (Table S3 in the ESI†). The net dipole moment for a unit cell was calculated to be a relative small value of 13.44 D. Hence, in compound 2, the weak SHG response could be mainly attributed to three factors. Firstly, due to the little distortions, it is very weak of the contributions from GaO4 tetrahedra. Secondly, the BO4 groups produce small second-order susceptibility. And lastly, the polarizations produced by BO4 and BO3 groups largely cancel each other out.
Ferroelectric and piezoelectric properties
The ferroelectric property of Ba4Ga[B10O18(OH)5](H2O) (2) was investigated because the crystal structure is in a polar space group (Cc) required for ferroelectric behavior. Ferroelectric measurements on pellets for Ba4Ga[B10O18(OH)5](H2O) (2) (5 mm diameter and 0.4 mm thick) showed ‘polarization loops’ which were frequency dependence and ferroelectric measurements revealed a very small remanent polarization (Pr) of 0.10 μC cm−2 and a saturation spontaneous polarization (Ps) of 0.20 μC cm−2 (Fig. S8 in the ESI†). Because these coefficients are very small, its ferroelectric property is negligible.5d After the sample was poled at electric field of 1.5 Ec, piezoelectric property of 2 was investigated and piezoelectric coefficient d33 was measured to be 3 pC N−1.Theoretical studies
To further understand the electronic structures of both compounds, theoretical calculations based on DFT methods were performed. The calculated band structures of β-BaGa[B4O8(OH)](H2O) (1) and Ba4Ga[B10O18(OH)5](H2O) (2) along high symmetry points of the first Brillouin zone are plotted in Fig. 4, and the state energies (electronvolts) of the lowest conduction band (LCB) and the highest valence band (HVB) of both compounds are listed in Table S4.† For the compound 1, the minimum of LCB is localized at G point, whereas the maximum of HVB is localized between Z and G point, displaying an indirect band gap of 5.17 eV. For compound 2, the minimum of LCB is localized at G point and the maximum of HVB is localized between E and C point, revealing an indirect band gap of 4.29 eV. The calculated band gaps are close to experimental values (4.65 and 4.12 eV for 1 and 2, respectively). | ||
| Fig. 4 Calculated band structure of β-BaGa[B4O8(OH)](H2O) (1) (a) and Ba4Ga[B10O18(OH)5](H2O) (2) (b). | ||
The bands can be assigned according to the total and partial DOS, as plotted in Fig. 5. We take β-BaGa[B4O8(OH)](H2O) (1) as a representative to describe them in detail, owing to the similarity between the two compounds. For compound 1, the valence band ranging from −21.0 to −16 eV arises from mostly O-2s, mixing with a small amount of B-2s2p and H-1s states. The band around −13 eV is mostly contributed from Ga-3d states, and the band around −10 eV is mostly contributed by Ba-5p. In the vicinity of the Fermi level, namely, from −10.5 to 0 eV in the valence band and from 4.8 to 13.6 eV in the conduction band, the O-2p, B-2s2p, Ga-4s4p, and H-1s states are all involved and overlap fully among them.
Population analyses give more information about quantitative bond analysis. The calculated bond orders of Ga–O, H–O bonds are 0.24–0.37 e, 0.58–0.61 e and 0.66–0.90 e, 0.34–0.65 e for B–O in BO3 and BO4 groups, respectively, for β-BaGa [B4O8(OH)](H2O) (1), whereas for Ba4Ga[B10O18(OH)5](H2O) (2), the calculated bond orders are 0.36–0.45 e, 0.59–0.69 e for Ga–O, H–O bonds and 0.76–0.85 e, 0.49–0.70 e for B–O in BO3 and BO4 groups, respectively. So we can say that the B–O bonds are stronger than Ga–O bonds in two compounds.
Conclusion
In summary, two new barium galloborates, namely β-BaGa[B4O8(OH)](H2O) (1) and Ba4Ga[B10O18(OH)5](H2O) (2) have been prepared by changing starting materials and stoichiometric ratios, and structurally characterized. They adopt two different anionic open frameworks based on polymeric borate clusters and GaO4 (or [Ga2O8]10−) groups. Compound 1 displays a two-dimensional (2D) layer anionic framework composed of [B4O8(OH)]5− clusters and [Ga2O8]10− dimers. Compound 2 crystallizes in a polar space group Cc and features a 3D network composed of [B10O18(OH)5]11− clusters and [GaO4]5− tetrahedra forming large 1D tunnels based on Ga3B16 19 MRs that accommodate the Ba2+ cations and H2O molecules. Compound 2 shows a weak SHG response of 0.2 times that of KDP. The results of our studies indicate that even in a same system, subtle changes of reaction conditions can lead to many different phases with different structures. Our future research efforts will be devoted to the preparation of other boron-rich or gallium-rich metal galloborates with interesting structures and physical properties.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grants 21231006, 21373222, and 21001107).Notes and references
- (a) K. M. Ok, E. O. Chi and P. S. Halasyamani, Chem. Soc. Rev., 2006, 35, 710 RSC; (b) C. T. Chen and G. Z. Liu, Annu. Rev. Mater. Sci., 1986, 16, 203 CrossRef CAS.
- T. Sasaki, Y. Mori, M. Yoshimura, Y. K. Yap and T. Kamimura, Mater. Sci. Eng., R, 2000, 30, 1 CrossRef.
- (a) P. Becker, Adv. Mater., 1998, 10, 979 CrossRef CAS; (b) M. E. Hagerman and K. R. Poeppelmeier, Chem. Mater., 1995, 7, 602 CrossRef CAS; (c) B. C. Wu, D. Y. Tang, N. Ye and C. T. Chen, Opt. Mater., 1996, 5, 105 CrossRef CAS.
- M. Touboul, N. Penin and G. Nowogrocki, Solid State Sci., 2003, 5, 1327 CrossRef CAS.
- (a) M. S. Dadachov, K. Sun, T. Conradsson and X. D. Zou, Angew. Chem., Int. Ed., 2000, 39, 3674 CrossRef CAS; (b) Y. F. Li and X. D. Zou, Angew. Chem., Int. Ed., 2005, 44, 2012 CrossRef CAS PubMed; (c) H. X. Zhang, J. Zhang, S. T. Zheng and G. Y. Yang, Inorg. Chem., 2005, 44, 1166 CrossRef CAS PubMed; (d) J. H. Zhang, F. Kong and J. G. Mao, Inorg. Chem., 2011, 50, 3037 CrossRef CAS PubMed; (e) X. Xu, C. L. Hu, F. Kong, J. H. Zhang and J. G. Mao, Inorg. Chem., 2011, 50, 8861 CrossRef CAS PubMed.
- (a) S. C. Sevov, Angew. Chem., Int. Ed. Engl., 1996, 35, 2630 CrossRef CAS; (b) R. Kniep, H. Engelhardt and C. Hauf, Chem. Mater., 1998, 10, 2930 CrossRef CAS.
- (a) J. Zhou, W. H. Fang, C. Rong and G. Y. Yang, Chem.–Eur. J., 2010, 16, 4852 CrossRef CAS PubMed; (b) T. Yang, A. Bartoszewicz, J. Ju, J. L. Sun, Z. Liu, X. D. Zou, Y. X. Wang, G. B. Li, F. H. Liao, B. Martin-Matute and J. H. Lin, Angew. Chem., Int. Ed., 2011, 50, 12555 CrossRef CAS PubMed.
- H. A. Höppe, K. Kazmierczak, M. Daub, K. Förg, F. Fuchs and H. Hillebrecht, Angew. Chem., 2012, 124, 6359 (Angew. Chem., Int. Ed., 2012, 51, 6255) CrossRef.
- (a) S. C. Wang, N. Ye, W. Li and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8779 CrossRef CAS PubMed; (b) X. Yan, S. Y. Luo, Z. S. Lin, Y. C. Yue, X. Y. Wang, L. J. Liu and C. T. Chen, J. Mater. Chem. C, 2013, 1, 3616 RSC.
- T. Hu, C. L. Hu, F. Kong, J. G. Mao and T. W. Mak, Inorg. Chem., 2012, 51, 8810 CrossRef CAS PubMed.
- Z. H. Liu, P. Yang and P. Li, Inorg. Chem., 2007, 46, 2965 CrossRef CAS PubMed.
- R. W. Smith, M. A. Kennard and M. J. Dudik, Mater. Res. Bull., 1997, 32, 649 CrossRef CAS.
- R. W. Smith, C. H. Hu and C. D. DeSpain, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, i23 CAS.
- G. K. Abdullaev and K. S. Mamedov, Zh. Strukt. Khim., 1972, 13, 943 CAS.
- Z. Yang, X. L. Chen, J. K. Liang, Y. C. Lan and T. Xu, J. Alloys Compd., 2001, 319, 247 CrossRef CAS.
- (a) Z. Yang, X. L. Chen, J. K. Liang, M. He and J. R. Chen, Cryst. Res. Technol., 2004, 39, 634 CrossRef CAS; (b) Z. Yang, J. K. Liang, X. L. Chen, T. Xu and Y. P. Xu, J. Alloys Compd., 2001, 327, 215 CrossRef CAS.
- Z. Yang, J. K. Liang, X. L. Chen and J. R. Chen, J. Solid State Chem., 2002, 165, 119 CrossRef CAS.
- H. Park and J. Barbier, J. Solid State Chem., 2000, 154, 598 CrossRef CAS.
- Q. Wei, L. Li, L. Cheng, Q. Meng and G. Y. Yang, Dalton Trans., 2014, 43, 9427 RSC.
- L. Cheng, Q. Wei, H. Q. Wu, L. J. Zhou and G. Y. Yang, Chem.–Eur. J., 2013, 19, 17662 CrossRef CAS PubMed.
- (a) H. X. Zhang, J. Zhang, S. T. Zheng, G. M. Wang and G. Y. Yang, Inorg. Chem., 2004, 43, 4168 Search PubMed; (b) D. B. Xiong, J. T. Zhao, H. H. Chen and X. X. Yang, Chem.–Eur. J., 2007, 13, 9862 CrossRef CAS PubMed; (c) Z. E. Lin, J. Zhang and G. Y. Yang, Inorg. Chem., 2003, 42, 1797 CrossRef CAS PubMed.
- W. M. Wendlandt and H. G. Hecht, Reflectance Spectroscopy, Interscience, New York, 1966 Search PubMed.
- S. W. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798 CrossRef CAS PubMed.
- (a) CrystalClear, version 1.3.5, Rigaku Corp., Woodlands, TX, 1999 Search PubMed; (b) G. M. Sheldrick, SHELXTL, Crystallographic Software Package, version 5.1, Bruker-AXS, Madison, WI, 1998 Search PubMed; (c) A. L. Spek, PLATON, Utrecht University, Utrecht, The Netherlands, 2001 Search PubMed.
- (a) M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717 CrossRef CAS; (b) V. Milman, B. Winkler, J. A. White, C. J. Pickard, M. C. Payne, E. V. Akhmatskaya and R. H. Nobes, Int. J. Quantum Chem., 2000, 77, 895 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- J. S. Lin, A. Qteish, M. C. Payne and V. Heine, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 4174 CrossRef.
- (a) I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef; (b) N. E. Brese and M. ÖKeeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef.
- (a) C. T. Chen, Y. C. Wu and R. C. Li, J. Cryst. Growth, 1990, 99, 790 CrossRef CAS; (b) H. Yu, H. Wu, S. Pan, Z. Yang, X. Su and F. Zhang, J. Mater. Chem., 2012, 22, 9665 RSC; (c) Y. Yang, S. Pan, J. Han, X. Hou, Z. Zhou, W. Zhao, Z. Chen and M. Zhang, Cryst. Growth Des., 2011, 11, 3912 CrossRef CAS; (d) C. L. Hu, X. Xu, C. F. Sun and J. G. Mao, J. Phys.: Condens. Matter, 2011, 23, 395501 CrossRef PubMed.
- (a) H. Y. Li, H. P. Wu, X. Su, H. W. Yu, S. L. Pan, Z. H. Yang, Y. Lu, J. Han and K. R. Poeppelmeier, J. Mater. Chem., 2014, 2, 1704 RSC; (b) Y. J. Shi, S. L. Pan, X. Y. Dong, Y. Wang, M. Zhang, F. F. Zhang and Z. X. Zhou, Inorg. Chem., 2012, 51, 10870 CrossRef CAS PubMed; (c) H. K. Izumi, J. E. Kirsch, C. L. Stern and K. R. Poeppelmeier, Inorg. Chem., 2005, 44, 884 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic files in CIF format, simulated and experimental XRD powder patterns, dipole moment calculations, the calculated state energies of the L-CB and H-VB, hydrogen bond, IR spectra, UV spectra, optical diffuse reflectance, TGA and DSC curves, ferroelectric properties data, coordination environments around the Ba atoms for both compounds. CSD reference numbers 427785, 427786. See DOI: 10.1039/c4ra06564f |
| This journal is © The Royal Society of Chemistry 2014 |