A deeper look into sonic spray ionization
Abdil Özdemirb,
Jung-Lee Lina,
Yi Sheng Wanga and
Chung-Hsuan Chen*a
aGenomics Research Center, Academia Sinica, Taipei, Taiwan. E-mail: winschen@gate.sinica.edu.tw
bDepartment of Chemistry, Faculty of Arts and Sciences, Sakarya University, 54187 Esentepe, Sakarya, Turkey
First published on 29th October 2014
Abstract
Sonic spray ionization (SSI) has been explored as an ambient ionization method for mass spectrometric analysis of different compounds. It has been applied to the analysis of different groups of compounds, mostly small molecules. The work reported in this paper extends the ionization efficiency of SSI and application of SSI to different groups of compounds, mostly biomolecules, in an ion trap mass spectrometer. SSI does not use any power supply and produces both positive and negative ions simultaneously. The most important parameters in SSI are gas pressure, solvent flow rate and physical dimensions of the spray device. Although the first two parameters have been investigated by different groups, the effect of physical dimensions has not been reported in the literature. Silica capillary inner diameter (ID) and outer diameter (OD) and ID of the cone play important roles in a spray. The best explored dimensions for the silica capillary are 150–200 μm OD and 40–75 μm ID and 350 μm ID for the cone. These physical dimensions provide the best ionization efficiency and spectra of large proteins such as albumin can be obtained. The spectra of the samples can also be collected in water without adding any acid into the solution.
1. Introduction
Electrospray ionization (ESI)1 is an atmospheric pressure ionization technique used to produce gas-phase ions of different groups of compounds for their analysis by mass spectrometry. ESI converts solution-phase analytes into gas-phase ions at atmospheric pressure when an analyte solution is sprayed through a small capillary. The liquid is dispersed as a fine mist of droplets into an electric field.1 One of the most important advantages of ESI is its operation under atmospheric conditions, which makes it the choice ionization method for coupling to mass spectrometric detection for different separation techniques and also makes it easier for manipulation of the source. Although it is not always required, the spraying process is often aided by a nebulizing gas and an electric field difference created between the spray capillary and inlet of the mass spectrometer. The important step in this process is ion formation from droplets. Formation of gaseous ions from nanodroplets in ESI is generally assumed to follow either the ion evaporation model (IEM)2 or the charge residue model (CRM)3,4 depending mostly on the mass of the ion. These two ionization mechanisms are valid for all spray ionization techniques involving the application of electric potential. All the commercial mass spectrometers use a nebulizing gas. Gas pressure will push the sprayed droplets to the mass spectrometer entrance and will provide faster desolvation of droplets. Application of voltage to the capillary creates a Taylor cone and gas pressure does not affect the formation of this cone.Sonic spray ionization (SSI)5–8 is one of the techniques that does not require any voltage application. Another technique Kelvin Spray Ionization9 also does not require any voltage application but system produces voltage by itself. SSI is an external ion source for mass spectrometry. The first time SSI was introduced by Hirabayashi et al.5 in 1994. They reported this new type ion source for capillary electrophoresis/mass spectrometry and liquid chromatography/mass spectrometry. They observed the highest ion intensity when the gas flow reaches to the sonic speed around 3 L min−1 (gas velocity of Mach 1), thus they named this source as sonic spray ionization. Hirabayashi et al.6 proposed several ionization mechanisms for SSI and the most acceptable one is charge separation in the droplets depending on gas type. In a liquid, positive and negative ions form ion pairs and their concentrations are uniform. However, in the gas phase close to the surface of droplets this distribution shows difference because of the surface potential. Hirabayashi et al.6 also claimed that this potential difference on the surface of droplets depends on gas type. For example N2 gas creates more ions than the Ar gas. Therefore they assumed that depending on gas type, positive and negative ions will have different distributions in a small droplet (<100 nm). A sheath gas flow at sonic speed can create a statistical imbalance of charges in the droplets. During the sonic gas flow, droplet fissions create an uneven distribution of positive and negative ions due to concentration fluctuations in droplets and finally ions are created by CRM.
Another variant of SSI has been introduced by Cooks and coworkers.10 Their source based on the principle of sonic spray ionization and their source employs a coaxial fused-silica capillary and a simple pneumatic spray operated at extremely high nebulizing gas flow rates. In their following studies, they combined SSI with ESI and named as Electrosonic spray ionization (ESSI).11,12 They have reported that with the help of the supersonic gas flow, an ESSI source is able to keep the folded conformation of proteins and produce narrow peak widths and charge state distributions at lower charge states of multiply charged protein ions. Another variant of SSI is Cold Spray ionization which sprays into a liquid nitrogen cooled chamber to preserve non-covalent complexes.13
Eberlin et al.14 used SSI to study desorption ionization as in DESI.15 Their technique was initially termed as desorption sonic spray ionization (DeSSI). In their subsequent studies, DeSSI was renamed easy ambient sonic spray ionization (EASI).14,16–18 Although the first versions of SSI method requires a high backpressure to achieve the sonic velocity of the nebulizer gas stream, Eberlin et al.19 improved their source using a small gas container with less gas and lower pressure (3 L min−1). Zilch et al.20 investigated the charge separation in the aerodynamic breakup of micrometer-sized water droplets using electrospray, sonic spray, and a vibrating orifice aerosol generator. Their work mainly focused on determination of droplet size, charge formation, and velocity after transmission through a capillary interface. Another ionization method which is called solvent assisted inlet ionization (SAII) was introduced by Pagnotti et al.21,22 This method also doesn't require any voltage application to the spray part. Sample is introduced directly inside the heated capillary and ionization happens inside the capillary. This ionization method produce mass spectra similar to those obtained with electrospray ionization (ESI) for small molecules, peptides, and large proteins such as carbonic anhydrase.21
SSI is one of the softest ionization techniques and this has been already shown in literature.10 SSI usually produces droplets that have much lower charge numbers than those produced through ESI. One of the most important applications of SSI is the analysis of very labile compounds. As it is specified before, SSI does not require any voltage application and heating process for the ionization of compounds. Therefore, ions can be created with less internal energy than ESI by SSI. Basically this situation might create two results. Either the solution phase interactions might be carried into the gas phase or it will create clustering of analytes.10
The SSI source is an easily fabricated simple device, which is similar to an ESI source. As it is reported,5–8 no voltage application is required and only gas flow is necessary for ion formation. For multiply charged ions, charge state distribution shifts to lower charge state and makes visible to see lower charge state of proteins without interrupting the solution phase structures. In previous studies, SSI was generally introduced as a spray ionization source that requires very high gas flow, high solvent flow and needs high concentration of samples. In this study we are reporting an improved version of SSI. There are key parameters which have not been investigated in detail in literature. Those key parameters are mostly physical dimensions of SSI source. The main part of this study shows how to design and manipulate a SSI source, apply this new source to different group of samples and show this source is a really softer ionization source than ESI. Several other parameters like temperature, gas flow, concentration and distance were investigated to obtain the best signal conditions for this new design. We are showing in this work that SSI does not need too high gas pressure, solvent flow rate and high concentration of analytes in the samples.
2. Materials and methods
2.1. Instrumentation
Experiments have been performed by using homemade spray source. It is similar to the one that produced by Prosolia Inc. A cross-sectional view of the sonic spray ion source is shown in Fig. 1a and b. The spray consists of stainless steel union including a silica capillary in varying diameters (20–150 μm i.d. and 150–375 μm o.d.). The SS union having varying inner diameters (200–450 μm) is manufactured as a screw type and it can be screwed into the peek main body of spray. Silica capillary is positioned in the center of this SS union. Silica capillary length is 5 cm and inner diameter and outer diameters were variable. By screwing the union in and out, spray profile can be manipulated. The tip of silica capillary was positioned in different location of the stainless steel fittings. The spray parts could be manipulated using a manual XYZ translational stage. The spray solvent was provided using a Cole Parmer 74900 Series syringe pump. Data were collected in full scan mode using Bruker Daltonics Data Analysis Version 3.2 software.Mass spectra were collected with an Esquire 3000 spectrometer (Bruker Daltonics Inc.) equipped with a quadrupole ion trap (QIT) mass analyzer. The front cover of the ionization chamber door was removed. The distance between the fused-silica capillary tip and the sampling orifice of the mass spectrometer was 5 mm, and their center axes were 1 mm off from mass spectrometer entrance. There was no potential difference between the capillary and the sampling orifice. Nitrogen gas (N2) was used as a nebulizing gas at a pressure of 15 to 150 psi to aid droplet formation and ionization. The most efficient sample flow rate was determined depending on the size of silica capillary. Liquid sample was introduced at a flow rate in the range of 0.25–1000 μl min−1. Electrospray spectra were recorded using the same ion source operated in a pneumatically assisted electrospray mode. Experiments for the comparison of ESI and SSI were carried out without changing source geometry and instrumental settings, except for the spray high voltage and nebulizing gas pressure.
For SSI experiments, protein samples with different solvents, concentrations and pH values were sprayed into the mass spectrometer by applying different amount of gas pressures. Alignment was carried out by maximizing the signal intensity of myoglobin in 0.1% acetic acid containing MeOH/water (1/1) solution. For other experiments different solvent compositions were used and for each case spray source was kept in the same position. For ESI measurements, potential difference of 4 kV was applied between spray and mass spectrometer entrance.
2.2. Reagents
All chemicals and solvents were of the highest commercial grade and used without further purification. Reagents including reduced glutathione (GSH) (307 Da), all the aminoacids, myoglobin (16.9 kDa), albumin (66.3 kDa), and insulin (5.7 kDa), were obtained from Sigma Aldrich (St. Louis, MO). Stock solutions of reagents were prepared at a concentration of 10−4 M in deionized (DI) water and further diluted with methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture with the addition of different amount of CH3COOH for positive ion spectra. The de-ionized water used for sample preparation was produced by a Milli-Q Gradient A10 water purification system with a Q-Gard®2 and a QuantumTM EX. High-performance liquid chromatography grade methanol, and acetic acid were purchased from Sigma chemical company.
water mixture with the addition of different amount of CH3COOH for positive ion spectra. The de-ionized water used for sample preparation was produced by a Milli-Q Gradient A10 water purification system with a Q-Gard®2 and a QuantumTM EX. High-performance liquid chromatography grade methanol, and acetic acid were purchased from Sigma chemical company.
3. Results and discussions
The experimental setup is shown in Fig. 1a and b. It includes a stainless steel (SS) union and a silica capillary. A nitrogen gas line is connected to the source part. SS cone part front of the main peek part plays an important role for ion formation. One side of the cone can be screwed into the peek main part of the spray (Fig. 1b). Inner diameter of the cone is in variable dimensions for the best optimizations. The gas flow is the main driving force in SSI and it is purged into the peek part perpendicularly to the capillary located in the middle part of source. While gas is travelling inside the source part, it starts making a circular motion and gas starts pushing the capillary to move in a circular orbit. Frequency of this motion depends on gas pressure but this vibration does not contribute to the ion formation. This motion basically decreases the ion intensity instead. The length of silica capillary is fixed and by screwing the SS cone in and out of peek body, different degree of ionization can be created when the capillary tip is inside and outside of the cone.Fig. 1c and d compare the spray profiles of SSI and ESI. The same instrumental settings were used in both cases. The main differences for both cases are applied voltage and nebulizing gas pressure. Because of applied voltage, gas phase ion formation follows two different ionization mechanisms, CRM and IEM depending on molecular weight of analytes for ESI. In SSI, it is assumed that ionization follows CRM after statistical imbalance of droplets. The statistical charging model was proposed by Dodd23 and accepted as the mechanism for charged droplet formation in the thermospray method. Hirabayashi et al.5 proposed the same mechanisms for SSI. In this model, solvent is suddenly evaporated into very fine equal-sized droplets. In some of those droplets, the number of positive and negative ions is not equally distributed. The resulting charging is caused by microscopic fluctuations during the spray process. Depending on all those explanations we investigated the spray formation of SSI using different size of silica capillary and SS cone. Fig. 1e and f shows two different spray profile of SSI. Gas flow around the silica capillary does not follow a straight path. As it is explained before, gas comes out in a swirling shape and creates a vortex front of the capillary. This vortex creates an imbalance in droplets and positive and negative ions are created at same time. Ion intensity depends on uniformity of vortex. If the capillary start vibration during the gas flow, the vortex formation is disrupted and ion intensity goes down. There is one more detail in our design that enhances the ion intensity an order of magnitude. The silica capillary was positioned 1 mm off axis in front of the mass spectrometer entrance. In this configuration, droplets first hit the front part of capillary and create more charge imbalance and form higher ion signal intensity in spectra.
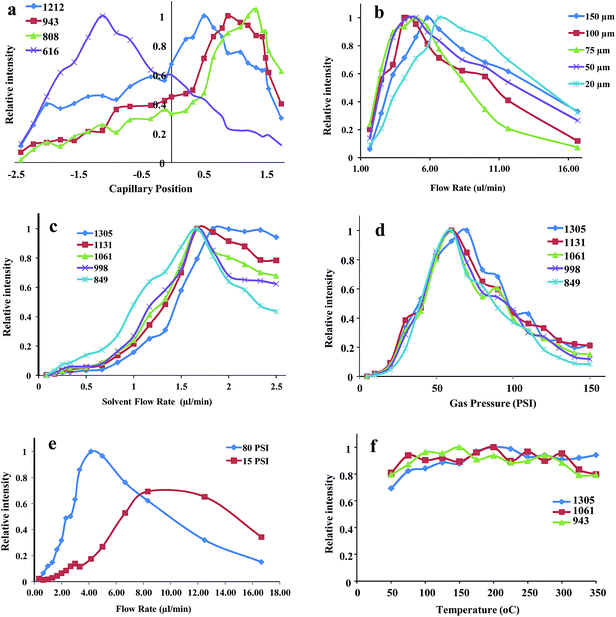
The tip of the capillary position inside the cone is an important parameter and changes the charge state distribution of samples a lot. Fig. 2a shows the signal intensity versus capillary tip position respect to the cone front end. The position of capillary tip was scanned through step by step inside the cone and signal intensity was recorded for each step. When the silica capillary tip is around 2.5 mm inside the cone, high mass ion intensity almost disappears and when the tip position slowly moves to front part of the cone signal intensity increases step by step. Interestingly the singly charged heme group signal increases more than that of high mass apomyoglobin ion when the capillary tip is inside the cone. This is most probably due to the loss of droplets inside the capillary. While droplets are exiting the cone, they hit the inner surface of cone and they lose their charge imbalance and thus reach charge balance again. Basically they do not lose the entire charge imbalance, thus the charge distribution of multiply charged ions shifts to the lower m/z region. In Fig. 2a, different charge states of myoglobin is shown and one of them is singly charged heme group (m/z 616). Heme group signal intensity slowly goes up and reaches to a highest point when the capillary is 1 mm inside the cone. In further movement of silica capillary, charge state distribution of myoglobin also shifts to lower m/z region and shows the highest intensity when the silica is around 1.0 mm out of cone. For lower m/z charge state distribution, capillary tip should be positioned out of cone. Broader charge distribution of analytes is one of the advantages of movable cone part.
The silica capillary and SS cone dimensions are important parameters for our new design SSI. The physical dimensions of those parameters were investigated. Outer diameter of silica capillary affects the signal intensity at certain degree. The smaller the capillary outer diameter the higher the signal can be obtained. But while changing the silica capillary OD, cone ID should also be changed proportionally, otherwise capillary will not fit into the cone. The ratio between these two values (cone ID/silica OD) should be around 1.75 to 2.2 but this ratio does not cover whole ID and OD range. In this work different size of capillaries and cones were tested to obtain the highest signal intensity. The best signal was obtained when the cone ID is around 350 μm and silica OD is around 150–200 μm. The most probable reason for this case might be the gas flow aerodynamics around the capillary. Gas flow creates air bubbles inside the liquid which is called cavitation in front of the capillary. Keurentjes et al.24 studied cavitation effect on radical formation in liquids. Cavitation refers to the formation, growth and collapse of gas or vapor bubbles induced by a pressure variation. Cavitation can be created by surface tension or energy deposition and in our case it is created by surface tension and it is called hydrodynamic cavitation. Gas flow can create a cavitation in front of the silica capillary. This phenomenon was seen during the spray process. Collapsing bubbles release some energy and cause radical formation. In this case releasing energy may not cause any radical formation but the cavitations can induce the formation of charge imbalance on droplets.
Fig. 2b shows the effect of silica capillary ID to the signal intensity of selected myoglobin ion. In this experiment 375 μm OD and 20 to 150 μm ID silica capillary and 450 μm ID SS cone were used and capillary was positioned out of cone. The size effect on multiply charged myoglobin ion at m/z = 849 was investigated. When the ID of capillary become smaller, the signal profile shifts to higher flow rate region. Between 100–50 μm regions, the signal profiles are similar to each other and the relative intensity of peaks reaches the highest value around 2.5 μl min−1. If the size of capillary is lower or higher than this value, the maximum signal shifts to higher flow rate region. Actually the silica capillary size should be smaller than 375 μm for better ion intensity but to investigate the capillary ID effect OD should be 375 μm which is commercially available size. As we stated before the best signal intensity is observed when the silica capillary OD is around 150 to 200 μm. If the OD size is bigger than this region, an effective charge distribution will not be created. The most probable reason for this situation is the thickness of capillary and it does allow formation of a good vortex and cavity in front of the capillary.
In SSI, there are critical parameters that have to be investigated in our modified source. Although we have examined all kind of parameters, we did not give all of them in this work, only the important parameters are given including solvent flow rate, gas flow, concentration and temperature effect. Myoglobin was taken as a model compound to investigate the effect of key parameters. The critical parameters were investigated using 150 μm OD, 40 μm ID silica capillary and 350 μm ID SS cone.
3.1. Effect of solvent flow rate
Fig. 2c shows the effect of solvent flow rate on the signal intensity of myoglobin. To observe the charge state distribution, five different charge state of myoglobin monitored at 60 psi gas pressure. As it is seen in Fig. 2c, SSI actually does not need very high solvent flow rate when it is compared with literature values. Flow rate scale is very similar to ESI limits. Signal from the samples can be observed as low as 0.25 μl min−1. The studied range of solvent flow rate is 0.15 to 17 μl min−1 and the highest signal intensity is observed around 1.7 μl min−1. It is interesting to show myoglobin spectra at solvent flow rate of 0.25 μl min−1 and gas pressure at 15 psi. Fig. 3 shows myoglobin mass spectra taken at specified conditions. Although these two values are very low when we compare with literature values, we can still observe pronounceable myoglobin spectra. Total ion chromatogram (TIC) shown as an inset figure also proves that obtained signal is stable during the data collection time. In this part, optimized flow rate is different than what we reported in previous section for silica capillary ID study. When the silica capillary OD is bigger than 200 μm, it needs higher flow rates for high signal intensity. In Fig. 2a one of the interesting details is the change in charge state distribution. At higher solvent flow rates, charge distribution of myoglobin shifts to higher m/z region and this can be seen in the comparison of m/z 849 and m/z 1305. Thus, the higher the solvent flow rate, the lower the charge state distribution was observed.3.2. Gas pressure effect
As a main driving force in SSI, gas pressure is very critical parameter. Hirabayashi et al.6 already investigate the effect of gas type to the ionization efficiency and one of the reported best choices is nitrogen gas and the same gas was used in optimization experiments. Using myoglobin solution, the influence of gas pressure to the peak intensities of multiply charged ions was systematically studied. As seen in Fig. 1, gas is introduced into the SSI in perpendicular position and inside the source, gas starts swirling around the silica capillary till it goes out of source. This swirling motion creates a vortex in front of the silica capillary. The created gas back-pressure depends on the physical dimensions of SSI source. Fig. 2d shows the gas pressure effect on the signal intensity of five selected charge states of myoglobin. In optimization experiments, optimized pressure was found as 60 psi and further increase in gas pressure signal intensity goes down. Although 60 psi is optimized gas pressure to collect best mass spectra, we do not need very high gas pressure. Fig. 3 also shows pronounceable mass spectra at 15 psi gas pressure. Fig. 3 is one of the unique results of our new design SSI source and these combined values have never been. Fig. 2e shows at 15 psi gas pressure, how high ion signal intensity can be observed. At same gas pressure, signal intensity significantly enhances when the solvent flow rate is increased.From the results reported above sections gas pressure and solvent flow rate results raises up some questions. Does SSI really require a sonic gas flow for ionization? Depending on our results ionization is happening at almost every gas flow and solvent flow rates. Efficient ionization only requires proper spray configuration. There are also other parameters that affect the signal intensity at certain degree. One of the parameter is the temperature effect. Fig. 2f shows the temperature effect on the signal intensity of myoglobin charge state distribution. We scanned through the temperature from 40 to 350 °C and did not see too much signal intensity change. The similar result was also observed by Cooks et al.10 at low solvent flow rates (3 μl min−1). To measure the concentration dependency on signal intensity, a series of myoglobin and insulin solutions were prepared in the range of 10−8 to 10−5 M in equal volume of methanol-water mixture with addition of 0.1% acetic acid. The lowest limit of concentration reached was in the 10−8 M region (no figure was shown).
The capability of SSI for the detection of peptide and high molecular weight proteins was evaluated with a series of protein standards ranging from 5.3 to 66 kDa. Fig. 4 illustrates the positive ion spectra of SSI along with their corresponding de-convoluted mass for a series of samples. SSI shows lower charge-state distribution than ESI. This is true for all kind of proteins. Although signal intensity of SSI is 1.5 orders of magnitude lower than ESI in same m/z region, SSI gives broader charge-state distribution for multiply charged proteins. New design SSI makes possible to take mass spectra of big molecules. Fig. 4g and h show albumin spectra, which have not been reported in the literature using SSI technique. Both spectra show same albumin spectra in same solution, only difference between these two spectra is target mass number of mass spectrometer and 4 h is taken in extended mode of instrument. This also proves that SSI gives very large charge state distribution and allows taking spectra of very big molecules. All these results also proves that SSI is a softer ionization technique and gives the opportunity to investigate the molecules with less disrupted structures by adding less charges on them. Fig. 4b, d and f show spectra of same proteins taken in only water with very low charge state. Basically it is hard to ionize samples in water without adding any acid or other ionizing regents by SSI. This result also shows the ionization efficiency of this new SSI source and allows us to monitor very low charge state of proteins. Singly charged ions of insulin and triply charged ions for myoglobin and cytochrome c were observed. These results indicate that the singly charged protein molecules can be also observed, if one uses an instrument which can reach a higher m/z region.
A soft ionization technique basically carries the solution phase structures into the gas phase.25–31 There are different thoughts about gentle ionization techniques; some believes that the gas phase structure is completely different than solution phase and some believe that this structure is same. If there is a difference between these two phase structures, the most probable reason for this difference might be the internal energy that is transferred to the molecules during the ionization. Ion formation mechanisms might be the most important reason for the formation of different amount of internal energies on the molecule. It is already reported in the literature10,30,31 that SSI is softer ionization mechanisms than ESI and any other charge involved ionization mechanisms. Takats et al.10 estimated amount of internal energy after kinetic energy transfer to the ions during the ionization process. Depending on their calculations IEM should transfer more energy than the CRM to the molecules. This probability might be also increased when the charge amount on the molecules decreased. Positive or negative changes on the molecules might change the conformation of molecules in gas phase. Using this analogy for soft ionization, SSI is the best ion source to carry solution phase structures into the gas phase.
Based on the explanations about SSI, we investigated the non-covalent interactions between GSH and 20 amino acids. Different mol numbers of glutathione and amino acids were mixed to reach the equilibrium, and then the mixed solution was investigated by ESI and SSI. Fig. 5a and b show the glutathione (GSH)-tryptophan complex formation. Due to the presence of some non-specific complexes arising from glutathione or amino acids, the formula for dissociation constants of complexes was obtained by improving the published formula by Ding et al.32,33 The improved formula does not have too much difference, just a couple more parameter was added and the Kd values for each case calculated and summarized in Table 1. The derivation of formula is not given in this work; it is beyond the scope of this paper. The observed Kd values obtained from SSI is smaller than the one obtained from ESI results. The binding of the complexes was further examined by collision-induced dissociation (CID) in a 3D ion trap mass spectrometer (data is not shown). As the stability of the non-covalent bonds was much weaker than other covalent ones, the dissociation of non-covalent bonds was easy in the CID, which led to the molecular ions arising from GSH-AA complexes. These studies revealed that glutathione could non-covalently bind to amino acids and this can be observed by SSI better than ESI.
| Kd | ||
|---|---|---|
| Amino acid | SONIC | ESI |
| Tryptophan | 4.826 × 10−5 | 1.05 × 10−3 |
| Lysine | 6.168 × 10−5 | 1.18 × 10−4 |
| Histidine | 1.714 × 10−4 | 6.83 × 10−4 |
| Arginine | 1.859 × 10−4 | 4.37 × 10−4 |
| Asparagine | 4.749 × 10−4 | 1.50 × 10−2 |
| Methionine | 6.808 × 10−4 | 0.00 × 100 |
| Phenyalanine | 7.782 × 10−4 | 4.09 × 10−2 |
| Proline | 1.110 × 10−3 | 1.95 × 10−2 |
| Glutamine | 1.253 × 10−3 | 2.17 × 10−3 |
| Leucine | 1.365 × 10−3 | 0.00 × 100 |
| Tyrosine | 1.407 × 10−3 | 1.51 × 10−2 |
| Threonine | 1.605 × 10−3 | 0.00 × 100 |
| Isoleucine | 1.710 × 10−3 | 0.00 × 100 |
| Aspartic acid | 2.795 × 10−3 | 0.00 × 100 |
| Valine | 3.556 × 10−3 | 0.00 × 100 |
| Serine | 6.837 × 10−3 | 0.00 × 100 |
| Cysteine | 1.185 × 10−2 | 2.72 × 10−3 |
| Glycine | 0.000 × 100 | 0.00 × 100 |
| Alanine | 0.000 × 100 | 0.00 × 100 |
Ionizations efficiency of SSI also tested using only water. Spectra of same utilized protein samples were taken by dissolving in water without adding any acid. Although the signal intensity is two orders of magnitude lower than the one obtained by ESI, spectra are very promising and provide very low charge distributions. This allows us to study structure of proteins without changing the solution phase conformations. For insulin it is possible to see single charged ions (Fig. 4b). In myoglobin and cytochrome c case, the lowest charge state determined is +3, it might be also possible to see singly charged ions but mass spectrometer should allow monitoring the high m/z region for those molecules (Fig. 4d and f).
4. Conclusion
A modified version of SSI was introduced and used to study proteins, peptides and noncovalent interactions. The analytical performance of the technique, including sample consumption, and sensitivity is comparable to the reported SSI methods. The SSI technique is also robust and provides reproducible results. We have demonstrated that multiply charged ions can be analyzed with the improved SSI interface from μM solution concentration in water and acidified water/methanol solutions at nL min−1 solvent flow rates. Spectra of protein samples having very high molecular weight like albumin have never been reported before with SSI. It can be ionized using this improved version of SSI source. The gas pressure as low as at 15 psi, spectra of all kind of samples can be obtained. SSI technique produces ions with less internal energy than ESI, showing that ion formation mechanisms follow the charge residue mechanism. The lower internal energy provides the transmission of non-covalent interactions into the gas phase without disrupting them. SSI can be used to investigate non-covalent interactions and fragile molecular structures and this brings another choice to the biological studies for ionization of molecules. In comparison with conventional ESI, the new SSI method can produce wider charge state distribution.References
- J. B. Fenn, M. Mann, C. K. Meng, S. F. Wong and C. M. Whitehouse, Science, 1989, 246, 64–70 CAS.
- J. V. Iribarne and B. A. Thomson, J. Chem. Phys., 1976, 64, 2287–2294 CrossRef CAS PubMed.
- M. Dole, L. L. Mach, R. L. Hine, R. C. Mobley, L. P. Ferguson and M. P. Alice, J. Chem. Phys., 1968, 49, 2240–2249 CrossRef CAS PubMed.
- G. Schmelzeisen-Redeker, L. Bütfering and F. W. Röllgen, Int. J. Mass Spectrom. Ion Processes, 1989, 90, 139–150 CrossRef CAS.
- A. Hirabayashi, M. Sakairi and H. Koizumi, Anal. Chem., 1994, 66, 4557 CrossRef CAS.
- A. Hirabayashi, M. Sakairi and H. Koizumi, Anal. Chem., 1995, 67, 2878 CrossRef CAS.
- A. Erabayashi, Y. Hirabayashi, M. Sakairi and H. Koizumi, Rapid Commun. Mass Spectrom., 1996, 10, 1703–1705 CrossRef.
- Y. Hirabayashi, A. Hirabayashi, Y. Takada, M. Sakairi and H. Koizumi, Anal. Chem., 1998, 70, 1882–1884 CrossRef CAS PubMed.
- A. Özdemir, J. L. Lin, K. J. Gillig and C. H. Chen, Analyst, 2013, 138, 6913–6923 RSC.
- Z. Takats, S. C. Nanita, G. Schlosser, K. Vekey and R. G. Cooks, Anal. Chem., 2003, 75, 1514 CrossRef CAS.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Anal. Chem., 2004, 76, 4050 CrossRef CAS PubMed.
- J. M. Wiseman, Z. Takáts, B. Gologan, V. J. Davisson and R. G. Cooks, Angew. Chem., Int. Ed., 2005, 44, 913 CrossRef CAS PubMed.
- K. Yamaguchi, J. Mass Spectrom., 2003, 38, 473 CrossRef CAS PubMed.
- R. Haddad, R. Sparrapan and M. N. Eberlin, Rapid Commun. Mass Spectrom., 2006, 20, 2901 CrossRef CAS PubMed.
- Z. Takats, J. M. Wiseman, B. Gologan and R. G. Cooks, Science, 2004, 306, 471 CrossRef CAS PubMed.
- R. Haddad, R. Sparrapan and M. N. Eberlin, Anal. Chem., 2008, 80, 898–903 CrossRef CAS PubMed.
- R. Haddad, H. M. S. Milagre, R. R. Catharino and M. N. Eberlin, Anal. Chem., 2008, 80, 2744 CrossRef CAS PubMed.
- V. G. Santos, T. F. F. Regiani, G. Dias, W. Romao, J. L. P. Jara, C. F. Klitzke, F. Coelho and M. N. Eberlin, Anal. Chem., 2011, 83, 1375 CrossRef CAS PubMed.
- N. V. Schwab, A. M. Porcari, M. B. Coelho, E. M. Schmidt, J. L. Jara, J. V. Visentainer and M. N. Eberlin, Analyst, 2012, 137, 2537–2540 RSC.
- L. W. Zilch, J. T. Maze, J. W. Smith, G. E. Ewing and M. F. Jarrold, J. Phys. Chem. A, 2008, 112, 13352–13363 CrossRef CAS PubMed.
- V. S. Pagnotti, N. D. Chubatyi and C. N. McEwen, Anal. Chem., 2011, 83, 3981 CrossRef CAS PubMed.
- V. S. Pagnotti, E. D. Inutan, D. D. Marshall, C. N. McEwen and S. Trimpin, Anal. Chem., 2011, 83, 7591 CrossRef CAS PubMed.
- E. E. Dodd, J. Appl. Phys., 1953, 24, 73–80 CrossRef CAS PubMed.
- M. W. A. Kuijpers, D. van Eck, M. F. Kemmere and J. T. F. Keurentjes, Science, 2002, 298, 1969–1971 CrossRef CAS PubMed.
- V. Katta, A. L. Rockwood and M. L. Vestal, Int. J. Mass Spectrom. Ion Processes, 1991, 103, 129–148 CrossRef CAS.
- S. K. Chowdhury, V. Katta and B. T. Chait, J. Am. Chem. Soc., 1990, 112, 9012–9013 CrossRef CAS.
- T. A. Fligge, M. Przybylski, J. P. Quinn and A. G. Marshall, Eur. Mass Spectrom., 1998, 4, 401–404 CrossRef CAS.
- A. K. Ganguly, B. N. Pramanik, A. Tsarbopoulos, T. R. Covey, E. Huang and S. A. Fuhrman, J. Am. Chem. Soc., 1992, 114, 6559–6560 CrossRef CAS.
- K. J. Lightwahl, B. L. Schwartz and R. D. Smith, J. Am. Chem. Soc., 1994, 116, 5271–5278 CrossRef CAS.
- J. S. Gardner, R. G. Harrison, J. D. Lamb and D. V. Dearden, New J. Chem., 2006, 30, 1276–1282 RSC.
- T. Benijts, W. Gunther, W. Lambert and A. De Leenheer, Rapid Commun. Mass Spectrom., 2003, 17, 1866–1872 CrossRef CAS PubMed.
- Z. Y. Dai, Y. Q. Chu, B. Wu, L. Wu and C. F. Ding, Acta Pharmacol. Sin., 2008, 29(6), 759–771 CrossRef CAS PubMed.
- H. R. Zhang, G. Chen, L. Wang, L. Ding, Y. Tian, W. Jin and H. Zhang, Int. J. Mass Spectrom., 2006, 252, 1–10 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |