Synthesis, insecticidal activity, structure–activity relationship (SAR) and density functional theory (DFT) of novel anthranilic diamides analogs containing 1,3,4-oxadiazole rings†
Abstract
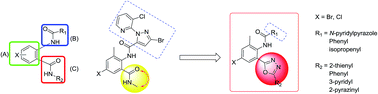
A series of anthranilic diamides analogs (5a–x) containing 1,3,4-oxadiazole rings were synthesized and characterized by 1H NMR, 13C NMR and mass spectrometry. The structure of N-(4-chloro-2-methyl-6-(5-(thiophen-2-yl)-1,3,4-oxadiazol-2-yl)phenyl)methacrylamide (5u) was determined by X-ray diffraction crystallography. The insecticidal activities of these new compounds against diamondback moth (Plutella xylostella) were evaluated. Preliminary bioassays indicated that some of these compounds exhibited good insecticidal activities against P. xylostella, especially 3-bromo-N-(4-bromo-2-methyl-6-(5-(pyrazin-2-yl)-1,3,4-oxadiazol-2-yl)phenyl)-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamide (5d), which displayed 100%, 80.95% and 57.14% activity against P. xylostella at 40 μg mL−1, 10 μg mL−1 and 4 μg mL−1, respectively. The relationship between structure and insecticidal activity was discussed. The density functional theory (DFT) studies could be helpful to understand the various insecticidal activities.

 Please wait while we load your content...
Please wait while we load your content...