Systematic investigation on charge storage behaviour of multidimensional poly(3,4-ethylenedioxythiophene) nanostructures†
Abstract
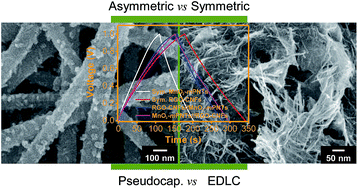
We provide in-depth insight into the electrochemical capacitive behaviour of multidimensional poly(3,4-ethylenedioxythiophene) (PEDOT) nanotubes (mPNTs) with unique surface substructures, such as nanonodules and nanorods (NR). NRs–mPNT had a capacitance of 153 F g−1 in acidic electrolyte, which corresponded to 73% of the theoretical maximum capacitance (210 F g−1). Moreover, they showed a 17% increase in specific capacitance when coupled to another pseudocapacitive component, namely, manganese dioxide (MnO2). MnO2–mPNTs were further tested in both symmetric and asymmetric cell configurations without using binders or conductive fillers, where reduced graphene oxide (RGO)–carbon nanofibers (CNFs) were employed as an electric double layer electrode material. The asymmetric MnO2–mPNTs (+)//RGO–CNFs (−) cell exhibited better performances than other asymmetric or symmetric cells of the MnO2–mPNTs/RGO–CNFs combination in terms of specific capacitance, cycling stability, and coulombic efficiency. At the same weight, the energy capacity of MnO2–mPNTs was similar to that of RGO–CNFs. The capacitive performance of asymmetric cells depended on the weight ratio of MnO2–mPNTs//RGO–CNFs. The optimized weight ratio of MnO2–mPNTs to RGO–CNFs in an asymmetric cell was 1 : 1. In terms of conductivity, chemical stability and solubility, PEDOT has superior advantages over other conducting polymers. It is expected that further optimization of electrode materials and cell systems will lead to the development of high-performance PEDOT-based electrochemical capacitors.

 Please wait while we load your content...
Please wait while we load your content...