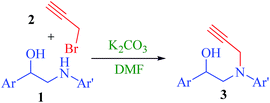
Copper catalysed [3 + 2] cycloaddition with concomitant annulation: formation of 2,4-diaryl-1,4-oxazepan-7-ones via a ketenimine route†
Selvam Kaladevi,
Arumugam Thirupathi,
Jeyaraman Sridhar and
Shanmugam Muthusubramanian*
Department of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai–625 021, Tamil Nadu, India. E-mail: muthumanian2001@yahoo.com; Fax: +91 452 2459845
First published on 12th August 2014
Abstract
A novel strategy for a copper catalyzed cascade reaction involving intramolecular nucleophilic addition to N-sulfonylketenimine gratifyingly furnishing 1,4-diaryl oxazepan-7-one has been described.
Introduction
Heterocycles act as synthons for potent pharmaceutical drugs and posses various biological activities. 1,4-Oxazepane derivatives represent an extensive group of crucial heterocyclic compounds, several of which find applications as dopamine D4 receptor ligands,1 fungal EF-2 inhibitors,2 telomerase inhibitors,3 glycosidase inhibitors,4 inhibitors of nitric oxide synthases5 and human A2A receptor antagonists.6 They also exhibit anti-fungal, antibacterial7 and anticonvulsant8 activities. The basic unit oxazepane is found in important natural products such as neurotoxin and batrachotoxin (Fig. 1).9 These factors ensure that 1,4-oxazepane derivatives are important synthetic targets for organic chemists.10 Popular methods available for the synthesis of 1,4-oxazepane derivatives includes reductive etherification,11 reductive amination,12 phosphine-triggered tandem [3 + 4] annulation reaction13 SN2-type ring opening reaction,14 solid-phase synthesis,15 liquid-phase synthesis,16 Cu(I)-catalyzed cycloaddition reaction17 and several multistep reactions.18Ketenimines are nitrogenated heterocumulenes that have attracted considerable interest due to their easy access and high reactivity getting themselves recognized as excellent precursors. The most attractive and sustainable method for the construction of ketenimines could be the copper catalyzed azide–alkyne cycloaddition reaction because of its mild reaction conditions.19 The ketenimines produced by the above protocol can be subjected to nucleophilic attack by amines, alcohols or water leading to amidines, imidates and amides respectively. It has been shown that substrates containing both a triple bond and a nucleophile, when added with sulfonyl azide, yield cyclic compounds.20 This phenomenon has been explored and applied in the assembly of various heterocycles.
Results and discussion
In continuation of our studies aiming at the development of efficient strategies for accessing privileged heterocycles from open chain compounds,21 it has been planned to make use of the reactivity of ketenimine functionality to generate new heterocyclic compounds. In this attempt, a seven membered ring, 2,4-diaryl-1,4-oxazepan-7-one nucleus, has been generated by an efficient one-pot procedure from easily available starting materials under mild conditions. An interesting cascade process, a nucleophilic addition followed by hydrolysis, has been observed during this reaction. Thus the journey for the new class of 2,4-diaryl-1,4-oxazepan-7-one derivatives started from the synthesis of 1-aryl-2-(phenyl(prop-2-ynyl)amino)ethanol 3 (Scheme 1), which was obtained by the reaction of reduced monophenacyl anilines 1 and propargyl bromide 2.The structure of the alkynes 3 was established from 1H, 13C and two dimensional NMR spectral data.
Ketenimines are known to react with nucleophiles yielding amidines. Accordingly, we examined the reaction of alkyne 3 with tolylsulfonyl azide 4 in the presence of copper iodide and triethylamine in dichloromethane at room temperature (Scheme 2). Instead of the anticipated N-(2,4-diphenyl-1,4-oxazepan-5-ylidene)-4-methylbenzenesulfonamide 5 (Scheme 2), 2,4-diphenyl-1,4-oxazepan-7-one 6 was obtained in good yield.
The optimized reaction conditions for the formation of 6b were summarized in Table 1. Among the several solvents and catalysts tested, dichloromethane in combination with copper(I) iodide appeared to be superior than the others. The desired product could be obtained in 42–90% yield using CH3CN, THF, DCE, toluene and CH2Cl2 as solvents. Dichloromethane has been found to be the solvent of choice. Other bases like pyridine and potassium carbonate resulted in lower yield of 6b. It has been found that the presence of copper catalyst in less than 10 mol% yielded the product quantitatively but required more time for the completion of the reaction (Table 1, entries 2 and 3). Addition of the catalyst in higher mole ratio has also not resulted in any improvement (Table 1, entry 1).
| Entry | Catalyst | Base | Solvent | Yield (%) | Reaction time (h) |
|---|---|---|---|---|---|
| 1 | CuI (20 mol%) | TEA | DCM | 90 | 0.5 |
| 2 | CuI (10 mol%) | TEA | DCM | 90 | 0.5 |
| 3 | CuI (5 mol%) | TEA | DCM | 85 | 3.0 |
| 4 | CuBr (10 mol%) | TEA | DCM | 83 | 0.5 |
| 5 | CuCl (10 mol%) | TEA | DCM | 65 | 0.5 |
| 6 | CuI (10 mol%) | K2CO3 | DCM | 57 | 5.0 |
| 7 | CuI (10 mol%) | Pyridine | DCM | 42 | 5.0 |
| 8 | CuI (10 mol%) | TEA | DCE | 70 | 1.0 |
| 9 | CuI (10 mol%) | TEA | THF | 68 | 1.5 |
| 10 | CuI (10 mol%) | TEA | CH3CN | 44 | 6.0 |
| 11 | CuI (10 mol%) | TEA | Toluene | 42 | 6.0 |
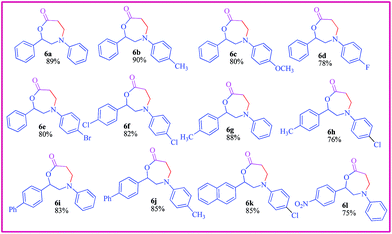
Sulfonyl azides with tosyl, mesyl and naphthyl groups also reacted well to give the respective 6 in excellent yields. Respective amines were isolated and identified in some cases. Differently substituted 1,4-oxazepane derivatives were obtained by employing differently substituted phenacyl bromides and anilines (Table 2). It is pertinent to note that in a related reaction, an interesting rearrangement has been observed.21b
| a Reaction conditions: p-toluenesulfonyl azide (1.2 mmol), 3 (1.0 mmol), TEA (2.0 mmol), copper(I) salt, solvent (10 mL), rt. |
|---|
 |
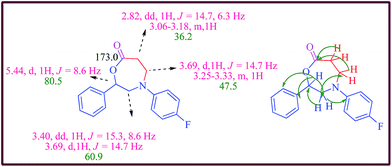
The structure of the oxazepanes was established from 1H, 13C and two dimensional NMR spectral data as illustrated for a representative example 6d (Fig. 2).
A plausible mechanism for the copper catalyzed intramolecular cyclization of electron deficient alkynes 2 is illustrated in Scheme 3. The reaction is believed to proceed initially through the ketenimine intermediate, generated by the addition of copper acetylide to sulfonyl azide, which is trapped by the nucleophilic centre present in the same compound. The intramolecular nucleophilic attack on the ketenimine central carbon results in the intramolecular ring closure followed by hydrolysis to the 2,4-diaryl-1,4-oxazepan-7-one (Scheme 3).
The possibility of initial propargylation at oxygen followed by cylisation can also be thought of. In that case, the final product is a seven membered lactam and not a lactone. However the HMBC connections and the fact that the IR spectrum 6e has a band at 1718 cm−1 clearly indicate that the compound formed is a lactone and not a lactam.
Conclusions
A copper-catalyzed cascade reaction involving intramolecular nucleophilic addition to N-sulfonylketenimine furnishing cyclic sulfonimidate, which subsequently undergoes hydrolysis, has been described. A seven membered ring, 2,4-diaryl-1,4-oxazepan-7-one nucleus, has been successfully constructed. The present method may be considered as a simple route for the synthesis of substituted 1,4-oxazepan-7-ones due to the short reaction time and readily available starting materials and catalyst.Acknowledgements
The authors thank CSIR major project, DST, New Delhi for assistance under the IRHPA program for providing funds for creating an NMR facility. Financial support from UGC-BSR-JRF to S. K is gratefully acknowledged.Notes and references
- K. Audouze, E. Q. Nielsen and D. Peters, J. Med. Chem., 2004, 47, 3089 CrossRef CAS PubMed.
- S. Kaneko, M. Arai, T. Uchida, T. Harasaki, T. Fukuoka and T. Konosu, Bioorg. Med. Chem. Lett., 2002, 12, 1705 CrossRef CAS.
- X. Liu, Y. Jia, B. Song, Z. Pang and S. Yang, Bioorg. Med. Chem. Lett., 2013, 23, 720 CrossRef CAS PubMed.
- P. A. Burland, H. M. I. Osborn and A. Turkson, Bioorg. Med. Chem., 2011, 19, 5679 CrossRef CAS PubMed.
- K. Shankaran, K. L. Donnelly, S. K. Shah, C. G. Caldwell, P. Chen, W. K. Hagmann, M. MacCoss, J. L. Humes, S. G. Pacholok, T. M. Kelly, S. K. Grant and K. K. Wong, Bioorg. Med. Chem. Lett., 2004, 14, 5907 CrossRef CAS PubMed.
- D. J. St. Jean and C. Fotsch, J. Med. Chem., 2012, 55, 6002 CrossRef CAS PubMed.
- S. Chandrasekhar, M. Seenaiah, A. Kumar, C. R. Reddy, S. K. Mamidyala, C. G. Kumar and S. Balasubramanian, Tetrahedron Lett., 2011, 52, 806 CrossRef CAS PubMed.
- G. Sharma, J. Y. Park and M. S. Park, Bioorg. Med. Chem. Lett., 2008, 18, 3188 CrossRef CAS PubMed.
- T. J. Grinsteiner and Y. Kishi, Tetrahedron Lett., 1994, 45, 8333 CrossRef.
- M. T. Crimmince and A. L. Choy, J. Am. Chem. Soc., 1999, 121, 5653 CrossRef.
- S. J. Gharpure and J. V. K. Prasad, Eur. J. Org. Chem., 2013, 2076 CrossRef CAS PubMed.
- S. K. Das, A. K. Srivastava and G. Panda, Tetrahedron Lett., 2010, 51, 1483 CrossRef CAS PubMed.
- R. Zhou, J. Wang, C. Duan and Z. He, Org. Lett., 2012, 14, 6134 CrossRef CAS PubMed.
- M. K. Ghorai, D. Shukla and K. Das, J. Org. Chem., 2009, 74, 7013 CrossRef CAS PubMed.
- J. Xu and X. Huang, J. Comb. Chem., 2009, 11, 938 CrossRef CAS PubMed.
- R. Racker, K. Doring and O. Reiser, J. Org. Chem., 2000, 65, 6932 CrossRef PubMed.
- S. Chandrasekhar, M. Seenaiah, A. Kumar, C. R. Reddy, S. K. Mamidyala, C. G. Kumar and S. Balasubramanian, Tetrahedron Lett., 2011, 52, 806 CrossRef CAS PubMed.
- (a) G. Sharma, J. Y. Park and M. S. Park, Bioorg. Med. Chem. Lett., 2008, 18, 3188 CrossRef CAS PubMed; (b) P. A. Burland, H. M. I. Osborn and A. Turkson, Bioorg. Med. Chem., 2011, 19, 5679 CrossRef CAS PubMed; (c) L. D. S. Yadav, V. P. Srivastava and R. Patel, Tetrahedron Lett., 2009, 50, 1423 CrossRef CAS PubMed; (d) K. Shankaran, K. L. Donnelly, S. K. Shah, C. G. Caldwell, P. Chen, W. K. Hagmann, M. MacCoss, J. L. Humes, S. G. Pacholok, T. M. Kelly, S. K. Grant and K. K. Wong, Bioorg. Med. Chem. Lett., 2004, 14, 5907 CrossRef CAS PubMed; (e) M. Bezanson, J. Pottel, R. Bilbeisi, S. Toumieux, M. Cueto and N. Moitessier, J. Org. Chem., 2013, 78, 872 CrossRef CAS PubMed.
- (a) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038 CrossRef CAS PubMed; (b) E. J. Yoo, M. Ahlquist, I. Bae, K. B. Sharpless, V. V. Fokin and S. Chang, J. Org. Chem., 2008, 73, 5520 CrossRef CAS PubMed; (c) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed, 2002, 41, 2596 CrossRef CAS.
- (a) K. Namitharan and K. Pitchumani, Org. Lett., 2011, 13, 5728 CrossRef CAS PubMed; (b) I. Yavari, M. Nematpour, S. Yavari and F. Sadegehizade, Tetrahedron Lett., 2012, 53, 1889 CrossRef CAS PubMed.
- (a) K. Rajaguru, R. Suresh, A. Mariappan, S. Muthusubramanian and N. Bhuvanesh, Org. Lett., 2014, 16, 744 CrossRef CAS PubMed; (b) M. Nagaraj, M. Boominathan, D. Perumal, S. Muthusubramanian and N. Bhuvanesh, J. Org. Chem., 2012, 77, 6319 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08008d |
| This journal is © The Royal Society of Chemistry 2014 |