Biomimetic star-shaped poly(ε-caprolactone)-b-glycopolymer block copolymers with porphyrin-core for targeted photodynamic therapy†
Abstract
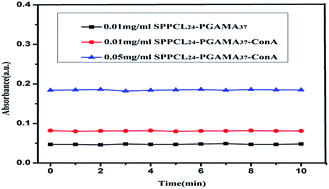
In this study, star-shaped porphyrin-cored poly(ε-caprolactone)-b-poly(gluconamidoethyl methacrylate) block copolymers (SPPCL-PGAMA) were successfully obtained. The synthetic route was via the ring-opening polymerization (ROP) of ε-caprolactone using a tetra-hydroxyethyl terminated porphyrin as a core initiator followed by the atom transfer radical polymerization (ATRP) of unprotected gluconamidoethyl methacrylate (GAMA) in 1-methyl-2-pyrrolidinone (NMP) solution at room temperature. The structure of the copolymer was thoroughly studied by nuclear magnetic resonance spectroscopy (NMR), Gel Permeation Chromatography (GPC), Fourier transform infrared spectroscopy (FT-IR) and differential scanning calorimetry (DSC). Notably, the as-prepared SPPCL-b-PGAMA that formed different structures being used for drug delivery systems has been researched. Moreover, this copolymer can release singlet oxygen under light irradiation and the singlet oxygen could be used for photodynamic therapy. In particular, UV-vis analysis showed that SPPCL-b-PGAMA has a very specific recognition with Concanavalin A (Con A) which provides porphyrin-cored SPPCL-b-PGAMA block copolymers for targeted drug delivery.

 Please wait while we load your content...
Please wait while we load your content...