Improved hydrogen production from formic acid under ambient conditions using a PdAu catalyst on a graphene nanosheets–carbon black support†
Yu-ling Qinac,
Jian-wei Wangac,
Yao-ming Wuab and
Li-min Wang*ab
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Changchun 130022, Jilin, China. E-mail: lmwang@ciac.jl.cn
bChangzhou Institute of Energy Storage Materials&Devices, Changzhou 213000, Jiangsu, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China. E-mail: ylqin@ciac.jl.cn
First published on 30th June 2014
Abstract
Formic acid (FA) has great potential as a suitable liquid source for hydrogen and hydrogen storage materials, provided highly active and selective dehydrogenation catalysts under ambient conditions are developed. Here, well-dispersed bimetallic gold–palladium (PdAu) nanoparticles (NPs) grown on graphene nanosheets–carbon black (GNs–CB) composite supports are synthesized via a facile co-reduction method, wherein the GNs–CB composite support proved to be a powerful dispersion agent and a distinct support for the PdAu NPs. Interestingly, the resultant PdAu/GNs–CB catalyst manifests high selectivity and exceedingly high activity to complete the decomposition of FA at room temperature.
Introduction
Hydrogen is generally proposed to be an important energy vector to face the increasing level of energy crisis and environmental pollution due to its high energy density and efficiency with low environmental load.1 However, even after several decades of intensive exploration, hydrogen storage is still one of the most challenging barriers that impedes the implementation of the hydrogen-based economy. FA, as a major product of biomass processing, has attracted considerable attention as a suitable liquid source for hydrogen and as a potential hydrogen storage material due to its intrinsic advantages, including high energy density, nontoxicity, and easy recharging as a liquid (the availability of the existing infrastructure for gasoline and oil).2Over the past decades, homogeneous catalysts for FA decomposition have been intensively investigated and significant advances have been achieved.3 However, those homogeneous catalysts suffer more or less severe drawbacks such as easy deactivation, hard to separate and recycle, use of organic solvents, etc.4 To solve these problems, increasing interests have recently been devoted to heterogeneous catalyst.
Pd NPs have been reported to exhibit excellent catalytic activity toward FA decomposition. However, monometallic Pd is prone to be deactivated because of the adsorption of poisoning carbon monoxide intermediates.5 In many works, Au is employed to improve the performance of Pd nanocatalysts, which various supports are used to load NPs.5,6 For example, at elevated temperature (90 °C), PdAu NPs loaded on ED-MIL-101 (ref. 5a) or C–CeO2 support,5c and PdAu@Pd/C (ref. 5d) have been reported to exhibit enhanced catalytic activity. The initial reaction rates of 106,5a 113.5 (ref. 5c) and 82.6 (ref. 5d) molH2 molcatalyst−1 h−1 have been achieved. To date, highly selective and efficient PdAu catalysts which can be used to significantly improve kinetic properties for catalytic decomposition of FA under ambient temperature are necessary.
Aside from tuning the catalyst composition, adjusting and optimizing the interaction between the active metal phase and the support materials are key approaches to further improve catalytic performance.7 An ideally high performance support guarantees good dispersion of active catalyst particles, facile electron transfer, and favorable mass transport kinetics. The strategy of coupling CB and GNs to form a novel carbon-based composite support for NPs has been reported in our previous work.4c Here, we use GNs–CB as a super support to load PdAu NPs, and graphene oxide (GO) is employed as the precursor of GNs. The resultant PdAu/GNs–CB catalyst exhibits improved catalytic performance toward complete decomposition of FA at room temperature. The prepared PdAu/GNs–CB nanocatalysts are also used to study the role that sodium formate plays in FA decomposition reaction.
Experimental section
Graphene oxide (GO) preparation
GO was made by a modified Hummers method. Briefly, graphite powder (3 g, 325 mesh) was put into an 80 °C solution of concentrated H2SO4 (12 mL), K2S2O8 (2.5 g), and P2O5 (2.5 g). After keeping at 80 °C for 4.5 h using a hot plate, the mixture was cooled to room temperature and diluted with 0.5 L of de-ionized water and left overnight. Then, the mixture was filtered and washed with de-ionized water using a 0.2 micron Nylon Millipore filter to remove the residual acid. The product was dried under room condition overnight. Next, the pretreated graphite powder was put into cold (0 °C) concentrated H2SO4 (120 mL) in a 250 mL round-bottom flask equipped with a magnetic stir bar. 15 g KMnO4 was added gradually under stirring while the temperature of the mixture was kept below 20 °C. The solution was then stirred at 35 °C for 2 h. Afterwards, 250 mL of de-ionized water was added and the suspension was stirred for another 2 h. Subsequently, additional 0.7 L of de-ionized water was added. Shortly after that, 20 mL of 30% H2O2 was added to the mixture to destroy the excess of permanganate. The suspension was then repeatedly centrifuged and washed first with 5% HCl solution and then with water. Exfoliation of graphite oxide to GO was achieved by ultrasonication of the dispersion for 120 min.Catalyst preparation
For the first step, 150 mg of Vulcan XC-72 carbon powder was ultrasonically dispersed in 20 mL of water and subsequently mixed with 15 mL GO solution (2 mg mL−1), then the mixture was stirred/sonicated for 6 h. H2PdCl4 (0.05285 M) and specific atom proportion of gold element (KAuCl4, 0.0273 M) aqueous solution were added into the mixture, followed by adding 10 mL of NaBH4 (10 mg mL−1) solution after 1 h. The mixture was stirred for another 8 h at room temperature in order to deposit the NPs onto the support absolutely. Finally, the desired catalyst from the suspension was centrifuged and washed with the distilled water and then dried in vacuum at 80 °C overnight. The synthesises of PdAu/CB and PdAu/GNs were similar with that of PdAu/GNs–CB. PdAu/GNs was obtained by frieze drying. In this work, the ratio of CB/GO support in PdAu/GNs–CB or Pd(Au)/GNs–CB is 5/1, except the samples in the Fig. 6.Instrumentation
Transmission electron microscope (TEM) was performed using a FEI Tecnai G2 S-Twin instrument with a field emission gun operating at 200 kV. Mass analysis of the generated gases was performed using a ThermoStar™ gas analysis system GSD 320 mass spectrometer (detection limit minimum: Faraday < 20 ppm, C-SEM < 1 ppm, 1–100 amu), wherein argon gas is chosen as cleaning gas. Gas analysis of the generated gases was performed using a Techcomp GC 7900 gas chromatography Analyzer, wherein argon gas is chosen as carrying gas. The detection limit for CO was below 10 ppm. XPS spectra were obtained with an ESCALABMKLL X-ray photoelectron spectrometer using an Al Kα source. The liquid chromatogram was obtained by Shimadzu LC-20AB with RI detector (Shimadzu RID-10A), and Aminex HPX-87H column (Bio-Rad, 300 × 7.8 mm), using 0.5 mM H2SO4 as fluent at a flow rate of 0.7 mL min−1 at 323 K. Powder X-ray diffraction (XRD) patterns were collected on Bruker D8 Focus Powder X-ray diffractometer using Cu Kα radiation (40 kV, 40 mA). The electrochemical tests were carried out with a BioLogic VMP3 electrochemical workstation. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) measurements were performed on a TJA (Thermo Jarrell Ash) Atomscan Advantage instrument.Electrochemical characterizations for the catalysts
For preparation of the thin-film working electrode, the GC electrode was sequentially polished with 3 and 0.5 μm Al2O3 paste (mixed with Al2O3 powder and ultrapure water). After the mechanical pretreatment, the electrode was cleaned by sonication in ultrapure water. To prepare the working electrode, 5 mg of the catalysts was dispersed in diluted Nafion alcohol solution which contained 1000 μL of ethanol and 25 μL of nafion, and was sonicated for 1 h to obtain a uniform suspension. Next, 8 μL of the suspension was pipetted onto the flat glassy carbon electrode. The coated electrode was then dried at room temperature for 10 min. Electrochemical experiments were carried out in a standard three-electrode cell at room temperature. The working electrode was the thin-film electrode with catalysts. Pt foil and Ag/AgCl electrode were used as the counter and reference electrodes, respectively. All potentials in this report referred to Ag/AgCl. All electrolyte solutions were deaerated with high-purity nitrogen for at least 30 min prior to any measurement.FA decomposition reaction
Catalytic reactions were carried out at room temperature using a two-necked round bottom flask with one of the flask openings connected to a gas burette and the other for the introduction of FA (Fig. S1†). Catalytic decomposition reaction of FA for the release of hydrogen (along with carbon dioxide) was initiated by stirring the mixture of FA solution introduced via a pressure-equalization funnel and the aqueous suspension of the catalyst prepared as described above. The volume of reforming gas was monitored by using the gas burette.Results and discussion
Structural characterizations of the PdAu catalyst
As shown in Fig. S2,† in contrast to pristine CB (XC-72), the GO–CB composite support shows better dispersion in water and facilitates the subsequent synthesis of highly dispersed PdAu catalyst. The N2 adsorption–desorption isotherms of GO–CB is shown in Fig. S3,† and the specific surface area is measured to be 324 m2 g−1. From Table 1, we can find that the specific surface areas for CB and GNS are smaller than the composite support. Specially, for GNs (GO reduced by NaBH4), the specific surface area reduces terribly because of the aggregation. The difference of BET among the three supports may affect the average size and dispersion of the NPs.| Sample | Metal content (%) | Average size of NPs (nm) | BET for support (m2 g−1) |
|---|---|---|---|
| PdAu/CB | Au, 4.04; Pd, 0.87 | 3.89 ± 0.7 | 202 |
| PdAu/GNs–CB | Au, 4.34; Pd, 0.92 | 2.79 ± 0.7 | 304 |
| PdAu/GNs | Au, 5.98; Pd, 1.25 | 4.03 ± 0.8 | 37.8 |
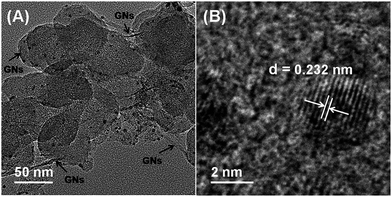
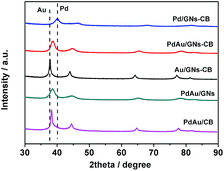
Fig. 1A shows the transmission electron microscopy (TEM) image of the as-synthesized PdAu/GNs–CB catalysts. CB and GNs can be differentiated obviously from Fig. 1A (CB is spherical; GNS is sheet like with wrinkle.). Clearly, GNs and CB are closely associated. As expected, well-dispersed PdAu NPs on GNs–CB were obtained. In the case of GO or CB alone under similar synthesis conditions, only larger PdAu NPs can be found (Table 1, average size of NPs are obtained from TEM micrographs (Fig. 1A and S4†)), highlighting the advantages in combining GNs and CB. The high-resolution TEM (HRTEM) image from Fig. 1B shows that the (111) d-spacing of Pd–Au nanoparticle is smaller than that of pure fcc-Au, but bigger than that of pure fcc-Pd. As shown in Fig. 2, X-ray diffraction (XRD) patterns of Au and Pd supported on GNs–CB both are in good agreement with the face-centered cubic metallic Au (PDF#65-2870) and Pd (PDF#46-1043), respectively. Because Au scatters X-rays more effectively than Pd, the diffractive peaks of Pd in PdAu NPs are hard to be detected. Furthermore, XRD patterns of bimetallic NPs reveal that all diffraction peaks are situated between those of Au and Pd, and thus, are reasonably indexed to PdAu alloy. The inductively coupled plasma atomic emission spectroscopy (ICP-AES) results show that the Au/Pd ratio of catalysts prepared with different support are 2.49, 2.54 and 2.57, respectively, which are almost in agreement with the ratio of metal precursors.
During the reduction process of Pd and Au precursors, GO could also be reduced by NaBH4. As shown in Fig. 3, C–C bonds (284.5 eV) become predominant while the peak of C–O is significantly reduced. However, it is worth noting that some residual oxygen-containing groups can still be found on the reduced GO, which could improve wettability and accessibility of aqueous FA solution to the active surface and improve the performance of the catalyst.8 In addition, Sharma8b also proved by experimental observations that the presence of residual oxygen groups on reduced GO plays an important role on the removal of carbonaceous species from the adjacent sites, which may be benefit for FA decomposition.
PdAu-catalyzed FA decomposition to generate hydrogen
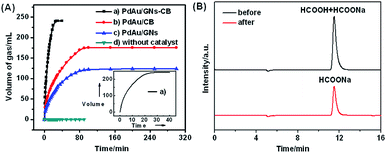
Fig. 4A shows the plots of volume of generated gas (CO2 + H2) versus the reaction time during dehydrogenation of aqueous FA solution catalyzed by PdAu NPs loaded on different supports. The illustration of apparatus for catalytic reaction is shown in Fig. S1.† FA decomposition or evaporation at room temperature without catalyst is not evident in Fig. 4A (trace d). From Fig. S5,† we can find that the prepared PdAu NPs without support show the poor catalytic activity toward FA decomposition at room temperature. Interestingly, the PdAu catalyst prepared in the presence of GO–CB composite support (a) exhibits enhanced activity in completing the decomposition of FA within only 30 min at room temperature in aqueous media (trace a and inset, Fig. 4A). Gas chromatograms (Fig. S6†) and mass spectral profiles (Fig. S7†) show that no detectable amount of CO (<10 ppm) is found in the gas mixture generated from the dehydrogenation of FA. Completion of the reaction is confirmed by the amount of released gas [n(H2 + CO2)/nHCOOH = 2.0] and the liquid chromatogram spectra (Fig. 4B, Sbefore ≥ 2Safter). The FA dehydrogenation performance at room temperature on PdAu/GNs–CB is much higher than the reported PdAu/ED-MIL-101 (reaction at 90 °C),5a PdAu/C–CeO2 (reaction at 90 °C),5c PdAu@Pd/C (reaction at 90 °C),5d and PdAu alloy NPs (reaction at 50 °C)5e catalysts. On the contrary, for the PdAu NPs synthesized with CB or GNs alone, only ∼80% (trace b, Fig. 4A) and ∼55% (trace c, Fig. 4A) of H2 are released from FA even after more than 5 h, which is much worse than that of PdAu catalyst prepared with GNs–CB. The values of the initial reaction rates (30 min) on PdAu/C and PdAu/GNs is 79.1 and 58.5 molH2 molcatalyst−1 h−1, which is much smaller than that on PdAu/GNs (175 molH2 molcatalyst−1 h−1).Regarding the improved catalytic performance of FA decomposition, we believe that PdAu/GNs–CB should have a much stronger tolerance to CO adsorption. CO stripping voltammetry is an effective method to determine the anti-poisoning ability of a catalyst toward CO. As shown in Fig. 5A and B, both the onset potential and the peak area of CO oxidation for PdAu/GNs–CB are much lower than those of the PdAu/CB catalyst, indicating that PdAu/GNs–CB catalyst possesses a strong anti-poisoning capability of CO.5d No visible CO oxidation peak for PdAu/GNs (Fig. 5C) is observed, revealing scarce CO absorption on the surface. Furthermore, cyclic voltammograms (CVs) (Fig. 5D) on the catalysts after reaction are employed. Clearly, a CO stripping peak can be found from the CV on PdAu/CB, which could be explained by the fact that CO from FA decomposition cannot be desorbed from the surface of the catalyst and thus reducing the activity. As for PdAu/GNs, although no CO absorbs on the surface of the catalyst, aggregation of GNs (Fig. S8†) decreases the catalytic activity.
The above mentioned results indicate that the enhanced activity of PdAu NPs supported on GNs–CB can reasonably be attributed to the combined effect of good dispersion and small particle size of PdAu NPs, as well as the anti-poisoning ability of the catalyst. However, since support plays an important role in catalytic performance, PdAu loaded on supports with different ratios of CB/GNs are also prepared. Their catalytic activities are shown in Fig. 6. Under the same PdAu loading condition, the best catalytic activity could be obtained when the ratio of CB to GNs is 5. Considering the above mentioned results, the poor activity of PdAu loaded on GNs–CB with other CB/GNs ratios may be related to the weak dispersion and/or anti-poisoning ability.
 | ||
| Fig. 6 Plot of completeness of FA decomposition over PdAu at different support ratios CB:GNs at 30 min. | ||
Bimolecular HCOOH/HCOO− mechanism on oxide catalysts model has been discussed by Borowiak.9 However, Zhou5c suggests that the mixture containing HCOOH and HCOO− keeps the NPs in a reduced state, consequently retaining the stability of the alloy catalyst. In order to elucidate the mechanism HCOONa involved in this dehydrogenation reaction, three additional experiments were performed in our work. No matter what decomposition mechanism the FA follows, C–H cleavage is the rate-determining step in obtaining hydrogen. Therefore, the concentration of formate ion may also be important for FA decomposition. To this end, Fig. 7A shows the plots of volume of generated gas (CO2 + H2) versus the reaction time during FA dehydrogenation at FA solution with different sodium formate concentration. Clearly, the reaction rate increases with increased concentration of formate. In this reaction system, sodium formate has two functions: increasing the pH value and the formate ion concentration. Hence, investigating the pH value separately is essential.
Fig. 8A shows the plots of volume of generated gas (CO2 + H2) versus the reaction time during FA dehydrogenation at different FA solution. Sodium acetate is used to replace sodium formate. As shown in Fig. 8A, only negligible product gas is observed from the decomposition of sodium formate solution (trace d, Fig. 8A). In the case of 2 M FA, only 15% FA is decomposed in pure FA solution during the first 60 min (trace b, Fig. 8A, pH = 1.87). No FA further decomposes even if the reaction time is extended to 210 min. For FA–sodium acetate solution (pH = 4.02), FA decomposes completely within 3 h (trace a, Fig. 8A) can be confirmed by liquid chromatography (Fig. 8B). It should be noted that although sodium acetate is added into 2 M FA solution after reaction (60 min) to increase the pH, almost no improvement in reaction activity is achieved (trace c, Fig. 8A), indicating that degradation of the catalyst in low pH cannot be recovered by subsequently increasing the pH. That is, highly efficient hydrogen production from FA decomposition can be achieved at room temperature with the help of PdAu/GNs–CB, provided that pH is sufficiently high. Again, in order to further confirm this point, FA solution (1 M FA and 1 M sodium formate) decomposition with different H+ concentration is shown in Fig. 7B. As the input of H+ is increased, the dehydrogen rate decreases obviously, which confirms that the higher pH value is benefit for the FA decomposition.
Lifetime is very important for the practical application of nanocatalysts. In this sense, recycle test of the PdAu/GNs–CB catalyst has been conducted for the same decomposing reaction. Repeated testing was obtained by adding pure FA (4 mmol) into the reaction vessel after the completion of the previous run. From Fig. 9, only a slight deactivation has been observed in the second run, which denotes good stability of PdAu/GNs–CB during the first two runs. Unfortunately, the catalytic activity highly decreases in the third run and forth run (Fig. S9†). Therefore, further investigations are needed to improve the durability of PdAu/GNs–CB.
 | ||
| Fig. 9 Three repeated testings of GNs–CB supported PdAu (8 mg) in 4 mL of FA solution at room temperature. | ||
Conclusion
In summary, a facile method is used to synthesize well-dispersed PdAu NPs grown on a GNs–CB composite support to combine the advantages of GNs and CB. Unexpectedly, PdAu NPs loaded on GNs–CB exhibit higher catalytic performance for FA decomposition at room temperature in aqueous media than on GNs or CB alone. Furthermore, the as-prepared PdAu NPs also exhibit high hydrogen selectivity in FA decomposition reaction that no detectable amount of CO is found in the gas mixture generated from the dehydrogenation of FA. Sodium formate also has an important function in promoting decomposition. This study proposed composite support and could open an effective method to prepared nanocatalysts for hydrogen generation from renewable fuels such as FA.Acknowledgements
This research is financially supported by the National Nature Science Foundation of China (Grant no. 20111061).Notes and references
- (a) S. Jones, J. Qu, K. Tedsree, X. O. Gong and S. C. E. Tsang, Angew. Chem., Int. Ed., 2012, 51, 11275 CrossRef CAS PubMed; (b) Y. He and Y. Zhao, Phys. Chem. Chem. Phys., 2009, 11, 255 RSC; (c) Y. Yamada, T. Miyahigashi, K. Ohkubo and S. Fukuzumi, Phys. Chem. Chem. Phys., 2012, 14, 10564 RSC; (d) Y. L. Qin, X. B. Zhang, J. Wang and L. M. Wang, J. Mater. Chem., 2012, 22, 14861 RSC; (e) L. Dienberg, J. Haug, G. Rauhut and E. Roduner, Phys. Chem. Chem. Phys., 2013, 15, 5836 RSC.
- (a) Q. Y. Bi, X. L. Du, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, J. Am. Chem. Soc., 2012, 134, 8926 CrossRef CAS PubMed; (b) D. A. Bulushev, L. Jia, S. Beloshapkin and J. R. H. Ross, Chem. Commun., 2012, 4184 RSC; (c) Z. L. Wang, J. M. Yan, H. L. Wang, Y. Ping and Q. Jiang, Sci. Rep., 2012, 2, 598 Search PubMed; (d) R. Muralidharan, M. Mclntosh and X. Li, Phys. Chem. Chem. Phys., 2013, 15, 9716 RSC; (e) H. Jeon, S. Uhm, B. Jeong and J. Lee, Phys. Chem. Chem. Phys., 2011, 13, 6192 RSC.
- (a) B. Loges, A. Boddien, H. Junge and M. Beller, Angew. Chem., Int. Ed., 2008, 47, 3962 CrossRef CAS PubMed; (b) C. Fellay, P. J. Dyson and G. Laurenczy, Angew. Chem., Int. Ed., 2008, 47, 3966 CrossRef CAS PubMed; (c) S. Enthaler, J. V. Langermann and T. Schmidt, Energy Environ. Sci., 2010, 3, 1207 RSC.
- (a) S. Zhang, Ő. Metin, D. Su and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 3681 CrossRef CAS PubMed; (b) Z. L. Wang, J. M. Yan, Y. Ping, H. L. Wang, W. T. Zheng and Q. Jiang, Angew. Chem., Int. Ed., 2013, 52, 4406 CrossRef CAS PubMed; (c) Y. L. Qin, J. Wang, F. Z. Meng, L. M. Wang and X. B. Zhang, Chem. Commun., 2013, 49, 10028 RSC.
- (a) X. Gu, Z. H. Lu, H. L. Jiang, T. Akita and Q. Xu, J. Am. Chem. Soc., 2011, 13, 11822 CrossRef PubMed; (b) H. L. Jiang, S. K. Singh, J. M. Yan, X. B. Zhang and Q. Xu, ChemSusChem, 2010, 3, 541 CrossRef CAS PubMed; (c) X. Zhou, Y. Huang, C. Liu, J. Liao, T. Lu and W. Xing, Chem. Commun., 2008, 3540 RSC; (d) Y. Huang, X. Zhou, M. Yin, C. Liu and W. Xing, Chem. Mater., 2010, 22, 5122 CrossRef CAS; (e) Ő. Metin, X. Sun and S. Sun, Nanoscale, 2013, 5, 910 RSC; (f) D. A. Bulushev, S. Beloshapkin, P. E. Plyusnin, Y. V. Shubin, V. I. Bukhtiyarov, S. V. Korenev and J. R. H. Ross, J. Catal., 2013, 299, 171 CrossRef CAS PubMed.
- K. Tedsree, T. Li, S. Jones, C. W. A. Chan, K. M. K. Yu, P. A. Bagot, E. A. Marquis, G. D. W. Smith and S. C. E. Tsang, Nat. Nanotechnol., 2011, 6, 302 CrossRef CAS PubMed.
- (a) A. Bruix, J. A. Rodriguez, P. J. Ramírez, S. D. Senanayake, J. Evans, J. B. Park, D. Stacchiola, P. Liu, J. Hrbek and F. Illas, J. Am. Chem. Soc., 2012, 134, 8968 CrossRef CAS PubMed; (b) L. He, Y. Huang, A. Wang, X. Wang, X. Chen, J. J. Delgado and T. Zhang, Angew. Chem., Int. Ed., 2012, 51, 6191 CrossRef CAS PubMed; (c) J. Macht and E. Lglesia, Phys. Chem. Chem. Phys., 2008, 10, 5331 RSC; (d) A. Primo, A. Corma and H. Garcia, Phys. Chem. Chem. Phys., 2011, 13, 886 RSC.
- (a) M. Pumera, Chem. Soc. Rev., 2010, 39, 4146 RSC; (b) S. Sharma, A. Ganguly, P. Papakonstantinou, X. Miao, M. Li, J. L. Hutchison, M. Delichatsios and S. Ukleja, J. Phys. Chem. C, 2010, 114, 19459 CrossRef CAS.
- M. A. Borowiak, M. H. Jamróz and R. Larsson, J. Mol. Catal. A: Chem., 2000, 152, 121 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed illustration of apparatus, TEM and SEM characterization, GC and MS analyses of gas. See DOI: 10.1039/c4ra05379f |
| This journal is © The Royal Society of Chemistry 2014 |