Catanionic vesicles charged with chloroaluminium phthalocyanine for topical photodynamic therapy. In vitro phototoxicity towards human carcinoma and melanoma cell lines†
P. Castagnosa,
M. P. Siqueira-Mouraab,
P. Leme Gotoab,
E. Pereza,
S. Franceschia,
I. Rico-Lattesa,
A. C. Tedescob and
Muriel Blanzat*a
aLaboratoire IMRCP, UMR CNRS 5623, Université Paul Sabatier, 118 route de Narbonne, 31062 Toulouse cedex 9, France. E-mail: blanzat@chimie.ups-tlse.fr; Fax: +33 5 61 55 81 55
bDepartamento de Química, Laboratório de Fotobiologia e Fotomedicina, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto (FFCLRP), Universidade de São Paulo, Ribeirão Preto, SP, Brazil
First published on 11th August 2014
Abstract
The hydrophobic character of chloroaluminium phthalocyanine (ClAlPc) and its tendency to dimerize in aqueous media reduces its topical penetration as well as its photodynamic efficacy. Lactose-derived catanionic vesicles, spontaneously obtained by mixing oppositely charged surfactants, are proposed as an alternative to other drug delivery systems to tackle this difficulty. Spectrofluorimetry studies confirmed the good loading capacity of the catanionic vesicles. Dynamic light scattering experiments, in various physiological media, were carried out to evaluate the stability of the ClAlPc-loaded system. In vitro phototoxicity studies performed on both human carcinoma and melanoma cell lines with increased light doses that are commonly used in clinical trials, look promising for the success of photodynamic therapy using ClAlPc-loaded catanionic vesicles.
Introduction
Skin cancers, which represent almost half of all cancers in the United States, present high resistance to a plethora of treatment modalities, such as chemotherapy or immunotherapy. Indeed, researchers worldwide have refocused their efforts on developing novel and cutting-edge strategies of treatment, which could reduce both morbidity and mortality rates among patients who have skin cancers.1,2 Compared to other treatment modalities available to cure skin cancers, photodynamic therapy (PDT) offers some advantages such as possibility to treat multiple lesions simultaneously and also to treat patients unsuitable for surgery.3 Topical PDT consists in the dermal application of a photosensitizer (PS) followed by its activation by a laser, resulting in the destruction of the tumour after the formation of highly reactive oxygen species. However, there is a strong correlation between the PDT response and the pigmentation degree of the tumor. The pigment melanin has an antioxidant activity, reducing the cytotoxic effect of ROS generated by PDT4 and, besides, act as cellular protector by absorbing ultraviolet (UV) light,5 with maximum light absorption around 530 nm, filtering the incident light.4 The first photosensitizers used in clinical PDT protocols were derivatives and purified fractions of hematoporphyrin derivative,6 excited in the range of wavelength lower than 640 nm. So, their use in PDT is really ineffective to treat pigmented tumors.5,7 A second generation of photosensitizers has been developed as they can enhance the tissue light penetration, since they absorb strongly in the red visible region. They avoid the photoresistance of pigmented melanoma and show increased efficacy in PDT that become responsive in the treatment of pigmented tumors.8 Among them, the hydrophobic chloroaluminium phthalocyanine (ClAlPc), represented on Fig. 1, has demonstrated good photodynamic effect on neoplastic cells.4,6 Nevertheless, phthalocyanines have been reported to display a strong tendency to aggregate in water as a result of the large hydrophobic skeleton avoiding contact with the aqueous medium.9 This aggregation process can affect their photophysical and photosensitizing properties, and therefore their photodynamic action,10 by reducing the production of reactive oxygen species11. The challenge of this project then consists in enhancing the photodynamic effect of ClAlPc on cutaneous tissues and therefore to change its pharmacokinetic profile using competitive drug delivery systems.As demonstrated in the literature,12 it is recognized that the development of new drug delivery systems carrying classical photosensitizers may increase the advantages of PDT. Vesicles are interesting systems for drug delivery, due in particular to their ability to encapsulate either hydrophilic (in the core) or hydrophobic (in the vesicle membrane) drugs, controlling their degradation, release, and bioavailability. They present the particularity to be flexible in terms of synthesis. Indeed, parameters inducing physico-chemical properties, such as their charge or their hydrophilicity are easy to modify through the synthesis process. Among drug delivery systems, catanionic vesicles, i.e., mixtures of cationic and anionic surfactants, firstly reported by Kaler et al.13 become more and more studied14–19 due to their simple preparation techniques and great stability.
As previously described,20,21 we managed to form lactose-derived vesicles, with a pseudo-tricatenar catanionic surfactant TriCat (Fig. 2), by a simple and safe preparation in aqueous solution. Lactose derived polar head provides sufficient hydrophilicity to ensure great stability of the vesicles and to enhance their biocompatibility. In particular, the cytotoxicity of these catanionic vesicles has already been evaluated on keratinocytes,22 showing a high cytotolerance of these systems.
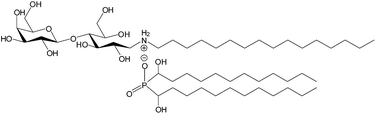 | ||
| Fig. 2 Chemical structure of the tricatenar catanionic surfactant derived from lactose named TriCat. | ||
Results and discussion
Preparation and characterization of ClAlPc loaded vesicles
The sugar-based tricatenar catanionic amphiphile TriCat that we developed (Fig. 2) results from a proton exchange between an amino-sugar derived surfactant, N-hexadecylamino-1-deoxylactitol, and bicatenar phosphinic acid amphiphile bis(hydroxydodecyl)phosphinic acid.20,23 As previously published, TriCat is characterized by the ability to spontaneously form stable vesicles in aqueous media above a critical aggregation concentration (CAC).20 These vesicles had a narrow distribution with a mean diameter of about 200 nm (see ESI Fig. S1†). Former freeze-fracture experiments proved a unilamellar structure for these vesicles.Catanionic vesicles have already proved to be able to incorporate, within their bilayer, amphiphilic fluorescent dyes22,24 and hydrophobic drugs,25,26 where the vesicles' properties are preserved. The incorporation of hydrophobic phthalocyanine ClAlPc in the TriCat catanionic vesicles' bilayers has been achieved after dissolution of the photosensitizer in acetone (see experimental part). In order to quantify the phthalocyanines encapsulation ratio, free phthalocyanines and encapsulated phthalocyanines, have been quantified using spectrofluorimetry (Fig. 3). Under these conditions, 92% (±2%) of ClAlPc have been encapsulated in catanionic vesicles, corresponding to a ratio of 8.7 × 10−4 mol ClAlPc per mol vesicle-forming molecule. This almost quantitative yield is largely superior to the ones obtained with other encapsulation systems, as only 63% of ClAlPc were encapsulated in nanocapsules and nanoemulsions.27
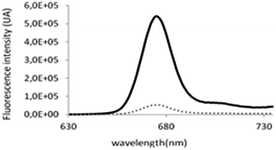 | ||
Fig. 3 Fluorescence intensities (λex: 610 nm) of (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) non-encapsulated ClAlPc and (−) total ClAlPc after vesicles destruction. ) non-encapsulated ClAlPc and (−) total ClAlPc after vesicles destruction. | ||
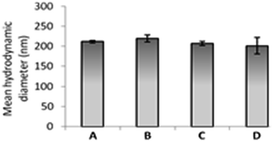
The size and the integrity of catanionic vesicles was verified using classical physicochemical techniques, as dynamic light scattering (DLS) and transmission electron microscopy (TEM). The vesicles obtained were matching (Fig. 4) those previously described without ClAlPc.20,22 A unique population centred on 200 nm was observed by both techniques. We also checked that addition of the neutral ClAlPc in the vesicles' bilayer has no influence on the charge of the particle already determined by zeta potential measurements at about −30 mV.24
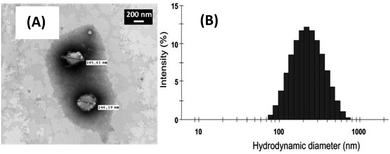 | ||
| Fig. 4 (A) TEM micrograph and (B) size distribution of catanionic vesicles of TriCat/ClAlPc prepared at 25 °C in water. | ||
Although absorption and fluorescence emission of ClAlPc are barely detectable in aqueous medium, previous studies have highlighted the recovering of these photophysical properties when ClAlPc is in hydrophobic domains of nanoemulsions, nanocapsules27 or even in liposomes' bilayers.28 Comparison of fluorescence intensities between free ClAlPc in water and ClAlPc encapsulated in catanionic vesicles showed a dramatic increase of the latest (Fig. 5). By analogy with previous studies, one can conclude that ClAlPc is encapsulated in the hydrophobic area of the catanionic vesicles' bilayer.
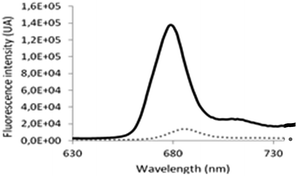 | ||
Fig. 5 Fluorescence intensities (λex: 610 nm) of (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) free ClAlPc in aqueous solution and of (−) ClAlPc encapsulated in catanionic vesicles. ) free ClAlPc in aqueous solution and of (−) ClAlPc encapsulated in catanionic vesicles. | ||
When considering the fluorescence emission spectra of ClAlPc (Fig. 3), the hypsochrome shift of the maximal fluorescence emission intensity at 685 nm for free ClAlPc in aqueous solution, to 674 nm in the presence of catanionic vesicles, confirms the incorporation of ClAlPc in a more confined environment, and thus its insertion inside the amphiphilic bilayer of the vesicles.29
Stability of catanionic vesicles charged with ClAlPc
Fig. 6 reports the stability over time of the samples described above in water. We can see that, the stability of the system was significantly increased in comparison with the stability of the system only composed of TriCat. Henceforth, in the presence of ClAlPc, vesicles' size distribution remained stable for more than 200 days.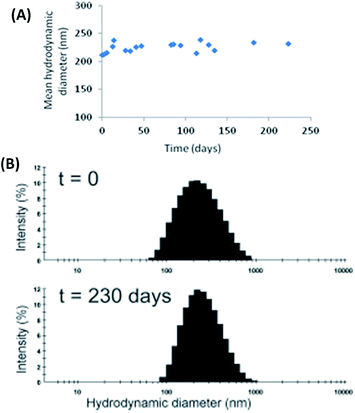 | ||
| Fig. 6 (A) Evolution over time of the mean hydrodynamic diameter and (B) size distribution (at t = 0 and t = 230 days) of TriCat/ClAlPc catanionic vesicles at 1 × 10−4 M in water at 25 °C. | ||
This great stability could probably be attributed to strong hydrophobic and Van der Waals forces between the lipidic chains of TriCat and the hydrophobic ClAlPc skeleton. In fact, similar explanation is proposed to explain cholesterol contribution to the cell membranes rigidity.30
Dilution stability of catanionic vesicles of TriCat/ClAlPc was then verified in water and in different physiological media. DLS experiments (Fig. 7) performed on a 1 × 10−4 M vesicular solution diluted to 2 × 10−5 M in water, PBS and cell culture media used (DMEM and RPMI) validated the great stability of these catanionic vesicular systems. Mean hydrodynamic diameters of 200 nm were obtained in all conditions, which still remained constant for more than 6 months.
Fluorescence studies on TriCat/ClAlPc catanionic vesicles before and after dilution highlighted that no modification of the ClAlPc environment occurred. No extinction of fluorescence was observed confirming that no ClAlPc was released from the vesicles. Dilutions of the TriCat/ClAlPc catanionic vesicles in the different physiological conditions do not modify the vesicles properties.
Physicochemical properties of TriCat/ClAlPc catanionic vesicles, in terms of size, encapsulation efficiency as well as their remarkable stability validate the great potential of these nanovectors as drug delivery systems. In vitro phototoxicity assays were then performed on two cancer cell lines involved in skin or oral squamous cell cancers.
Cytotoxicity studies of catanionic vesicles charged with ClAlPc
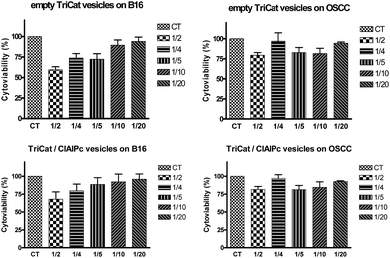
In order to validate the use of these loaded vesicles on cancer cells and determine the working concentration, the cytoviabilites have been evaluated. Five concentrations of catanionic vesicles were tested, on two different cancer cell lines (melanoma cells B16-F10 and carcinoma cells OSCC), obtained from dilutions in culture medium of mother solution formulated at 1 × 10−4 M in TriCat. Results from cytotoxicity assays of vesicles applied on B16-F10 and OSCC cell lines are presented on Fig. 8.Results have shown a light cytotoxicity of catanionic vesicles, whatever their nature and their concentration. The chosen concentration for the following experiments is 2 × 10−5 M in TriCat (containing 1 μg mL−1 of ClAlPc).
In vitro phototoxicity of ClAlPc loaded vesicles
The phototoxicity assays were carried out with TriCat/ClAlPc catanionic vesicles at respectively 2 × 10−5 M and 1.74 × 10−6 M (1 μg mL−1), since these concentrations did not show toxicity in darkness on either cell lines (B16-F10 and OSCC). The cellular viability results were obtained after irradiations with a laser at 670 nm, which corresponds to the maximum absorption wavelength of ClAlPc. The light doses used, up to 15 J cm−2 are well accepted in PDT studies with pigmented melanomas.8 Cells treated with catanionic vesicles loaded with ClAlPc illustrated a decrease of cell viability proportional to the increase of light dose (Fig. 9).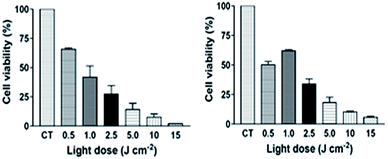 | ||
| Fig. 9 Cell viability of (A) B16-F10 melanoma cells and (B) OSCC cells pre-incubated with TriCat/ClAlPc catanionic vesicles and irradiated with a laser (λ = 670 nm) using increased light doses. | ||
The percentage of cell survival ranged from 65.8% (±1.06) to 1.9% (±0.17) for B16-F10 melanoma cells and from 50.2% (±2.77) to 5.7% (±0.99) for OSCC cells treated with the lowest and highest light dose, respectively. The results showed that B16-F10 cells were more sensitive to photodynamic damage (p < 0.05) compared to OSCC after irradiation using the highest light dose (15 J cm−2) (for details see ESI†). Such a finding could be attributed to B16-F10 cells be more susceptible than those OSCC to destruction by singlet oxygen and free radicals generated by PDT. For other light doses applied, there was no significant difference (p > 0.05) between the cell viability of both cell lines treated. Our findings are consistent with those from another study in which ClAlPc encapsulated into liposomes was used to treat human oral carcinoma cells (keratinocytes). A significant decrease in cell proliferation (80%) was achieved by combined application of ClAlPc liposomes at 2.9 μg mL−1 (5 × 10−6 M) and light dose 25 J cm−2.31 Moreover, concerning previous works performed on pigmented melanoma cells with other photosensitizer-loaded drug delivery systems, most of the articles report the use of much higher light doses, from 20 and 200 J cm−2 (ref. 32–34) to achieve the same phototoxic efficiency. Therefore, all the results from in vitro phototoxicity assay point to the potential of PDT using TriCat/ClAlPc which could be considered as a promising tool for therapy against both oral carcinoma and melanoma.
Conclusion
The results described in this paper validate an alternative vesicular system to liposomes for topical PDT. Indeed, we have prepared catanionic vesicles derived from lactose, which are easy to set-up. Catanionic vesicles were spontaneously obtained after dissolution of two surfactants in water above a critical aggregation concentration. Moreover, the encapsulation trials have demonstrated that they can entrap hydrophobic chloroaluminium phthalocyanine (ClAlPc) almost quantitatively. Physicochemical studies on ClAlPc-loaded vesicles have highlighted an increased stability of the system compared to the unloaded ones. They remained stable for months without any degradation to dilution. In vitro phototoxicity results on both B16-F10 and OSCC cell lines demonstrated that catanionic vesicles improve the anti-tumoral effect of ClAlPc with light doses currently used in clinical protocols encouraging further studies on TriCat vesicles loaded with different kinds of photosensitizer drugs.Experimental section
Preparation and physicochemical characterization of catanionic vesicles (TriCat and TriCat/ClAlPc)
The tricatenar catanionic surfactant TriCat, was obtained according to protocols already described in the literature.16,17A solution of chloroaluminium phthalocyanine (ClAlPc) in acetone (100 μg mL−1) was diluted in milli-Q water (5% v/v). As already described,22,24 freeze-dried TriCat (synthesis described in ESI† document) was put in the acetone–water solution (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) of ClAlPc at the concentration of 1 × 10−4 M in TriCat (which is above CAC (3 × 10−5 M)), stirred and then sonicated (Vibra Cell, Bioblock Scientific®, titanium probe, pulse rate: 30%, intensity: ×3) for 15 min. Acetone was removed under vacuum using a rotavapor (30 min, 50 mbar, 40 °C) until no acetone signal was detectable in 1H NMR.
95) of ClAlPc at the concentration of 1 × 10−4 M in TriCat (which is above CAC (3 × 10−5 M)), stirred and then sonicated (Vibra Cell, Bioblock Scientific®, titanium probe, pulse rate: 30%, intensity: ×3) for 15 min. Acetone was removed under vacuum using a rotavapor (30 min, 50 mbar, 40 °C) until no acetone signal was detectable in 1H NMR.
Sizes of catanionic vesicles obtained were determined using Dynamic Light Scattering (Malvern Instruments®, Nano ZS ZEN3600, UK). The analysis was performed with a He–Ne laser, a scattering angle of 173° and at a temperature of 25.0 °C ± 0.1 °C. Vesicles' size and morphology were verified by transmission electron microscopy using a JEOL® JEM 1011 electron microscope, operating at 120 kV.
Encapsulation efficiency of ClAlPc in TriCat vesicles was determined using the difference between the quantity of non-encapsulated phthalocyanine and total amount of phthalocyanine, measured by spectrofluorimetry.
| EE (%) = (1− cnon encapsulated ClAlPc/ctotal ClAlPc) × 100 |
The total quantity of ClAlPc was determined after vesicles' bursting, obtained by adding methanol to the solution (2 mL of methanol for 10 μL of vesicular solution). Non encapsulated ClAlPc concentration was determined after separation of aqueous phase using an AMICON device (Microcon®, molecular weight cut-off = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Millipore®) and filtration/centrifugation protocol: 1 h, 10 000 rpm, 4 °C. 10 μl of the ultrafiltrated obtained was diluted in 2 mL methanol.
000, Millipore®) and filtration/centrifugation protocol: 1 h, 10 000 rpm, 4 °C. 10 μl of the ultrafiltrated obtained was diluted in 2 mL methanol.
Spectrofluorimetry measurements were performed on vesicles of TriCat/ClAlPc, on a Spectrofluorimeter Hitachi F-4500. All the slit widths were set at 2 nm. The excitation wavelength was set at 610 nm and the emitted intensity was collected from 630 to 750 nm.
Physicochemical characteristics (shape, size and stability) of ClAlPc loaded vesicles were checked to be stable under dilution by Dynamic Light Scattering, transmission electron microscopy and tensiometry analyses, even at concentrations inferior to the surfactant CAC.20
Cell culture
Melanoma cell line (B16-F10) was grown in RPMI Medium (Gibco, Grand Island NY, USA) supplemented with 10% foetal bovine serum (FBS), 1% penicillin–streptomycin, and 0.625 μg mL−1 Amphotericin B (Gibco, Grand Island, NY, USA).Oral Squamous Cell Carcinoma (OSCC) was grown in DMEM Medium (Gibco, Grand Island NY, USA) supplemented with 10% FBS, 1% non-essential amino acids, 1% penicillin–streptomycin, and 0.625 μg mL−1 Amphotericin B (Gibco, Grand Island, NY, USA).
Both cell lines were grown at 37 °C with 5% CO2 inside a humidified incubator (80%) in darkness.
In vitro cytotoxicity assay
Cytotoxicity assay of TriCat vesicles and TriCat/ClAlPc vesicles was evaluated by tetrazolium-based colorimetric 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide (MTT) method.Both cell lines were seeded into 96-well plates (2 × 104 cells per well) in medium supplemented with 10% FBS 24 h before incubation with one of the three vesicles solutions. The cells were washed with 100 μL well−1 of Hank's Buffer Solution before being treated with vesicles formulated at 1 × 10−4 M in TriCat extemporaneously diluted until given concentrations in culture medium without FBS or with medium without FBS as a control solution. They were incubated during 3 h at 37 °C 5% CO2 humidified atmosphere in darkness. Subsequently, the medium with formulations was removed, then 100 μL mL−1 of fresh medium supplemented with 10% FBS was added. Cells were incubated overnight at 37 °C, 5% CO2, humidified atmosphere in darkness.
Medium was then removed and 200 μL well−1 of a MTT solution (composed of 30 μL of MTT at 5 mg mL−1 in Hank's Buffer Solution and 170 μL of medium without red phenol) was added. The plates were then incubated for 4 h at 37 °C (5% CO2, humidified atmosphere, darkness). Supernatant solutions were removed and the MTT-formazan product was solubilized in 200 μL well−1 of 2-propanol. After homogenization of the solutions, plates were analyzed by Microplate Reader Molecular Devices VERSA Max tunable and optic density was estimated at 560 nm and 690 nm.
Statistical analysis was performed using Prism 4.0® (GraphPad Software) by one-way ANOVA and Tukey test. All data were expressed as the mean ± SEM of three independent experiments obtained on samples provided by independent sources. Statistical significance for this study was considered at p < 0.05.
Five concentrations of catanionic vesicles were tested on cells obtained from dilutions in culture medium without FBS of mother solution formulated at 1 × 10−4 M in TriCat (from 5 × 10−5 M to 5 × 10−6 M).
In vitro photocytotoxicity assay
Photocytotoxicity assay of ClAlPc incorporated into catanionic vesicles was evaluated by tetrazolium-based colorimetric MTT method, as previously described.35Both cell lines were seeded into 24-well plates (1 × 105 cells per well) one day before light irradiation. Cells were treated with culture medium alone, as the control, and with the culture medium containing the vesicles in the concentration of 1 μg mL−1 ClAlPc. They were incubated during 3 h at 37 °C, 5% CO2, and humidified atmosphere in darkness. Subsequently, the medium with formulations was removed, and cells were rinsed with 500 μL per well of Hank's Buffer Solution, then 500 μL per well of fresh medium without phenol red was added.
Irradiations were performed at 670 nm in aseptic conditions with light dose adjusted for 0.5 to 15 J cm−2. The apparatus used was a laser-diode Eagle (Quantum Tech, São Carlos–SP, Brazil) at a power of 820 mW, with a density of 261 mW cm−2.
Medium was removed and replaced by 500 μL per well of fresh medium supplemented with 10% FBS. Cells were incubated overnight at 37 °C, 5% CO2, humidified atmosphere in darkness. Then, after removing medium, cells were washed with 500 μL per well of Hank's Buffer Solution. 500 μL per well of MTT solution (composed of 80 μL of MTT at 5 mg mL−1 in Hank's Buffer Solution + 420 μL of medium without red phenol) was added, and cells were incubated for 4 h at 37 °C (5% CO2, humidified atmosphere, darkness). Supernatant solutions were removed and the MTT-formazan product was solubilized in 1 mL per well of 2-propanol. After homogenization, solutions were transferred into 96-well plates (where 1 well of 24-well plate is equivalent to 4 wells of 96-well plate, 96-well plates being filled with 200 μL per well of solutions). Plates were analyzed by Microplate Reader Molecular Devices VERSA Max tunable and optic density was estimated at 560 nm and 690 nm.
Statistical analysis was performed using Prism 4.0® (GraphPad Software) by one-way ANOVA, and Tukey test. All data were expressed as the mean ± SEM of three independent experiments, obtained on samples provided by independent sources. Statistical significance for this study was considered at p < 0.05.
Acknowledgements
CNRS and University Paul Sabatier of Toulouse are acknowledged for institutional funding. A.C. Tedesco thanks financial support from the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES), the State of São Paulo Research Foundation (FAPESP), and the National Council for Scientific and Technological Development (CNPq). The authors also thank the USP/COFECUB agreement (FFCLRP-USP – Proc. 10.1.1085.59.1).Notes and references
- M. Villanueva and M. Herlyn, Curr. Oncol. Rep., 2008, 10, 439 CrossRef.
- J. C. Muhrer, J. Nurse. Pract., 2009, 5, 35 CrossRef PubMed.
- A. Sidoroff and P. Thaler, Photodiagn. Photodyn. Ther., 2010, 7, 24 CrossRef CAS PubMed.
- L. M. Davids and B. Kleemann, Cancer Treat. Rev., 2011, 37, 465 CAS.
- A. Slominski, D. J. Tobin, S. Shibahara and J. Wortsman, Physiol. Rev., 2004, 84, 1155 CrossRef CAS PubMed.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Ca-Cancer J. Clin., 2011, 61, 250 CrossRef PubMed.
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Photodiagn. Photodyn. Ther., 2004, 1, 279 CrossRef CAS.
- A. Kawczyk-Krupka, A. M. Bugaj, W. Latos, K. Zaremba and A. Sieroń, Photodiagn. Photodyn. Ther., 2013, 10, 503 CrossRef CAS PubMed.
- S. Dhami and D. Phillips, J. Photochem. Photobiol. Chem., 1996, 100, 77 CrossRef CAS.
- X. Damoiseau, H. J. Schuitmaker, J. W. Lagerberg and M. Hoebeke, J. Photochem. Photobiol. B Biol., 2001, 60, 50 CrossRef CAS.
- C. Tanielian and G. Heinrich, Photochem. Photobiol., 1995, 61, 131 CrossRef CAS PubMed.
- Y. Sadzuka, F. Iwasaki, I. Sugiyama, K. Horiuchi, T. Hirano, H. Ozawa, N. Kanayama and T. Sonobe, Int. J. Pharm., 2007, 338, 306 CrossRef CAS PubMed.
- E. W. Kaler, A. K. Murthy, B. E. Rodriguez and J. A. Zasadzinski, Science, 1989, 245, 1371 CAS.
- E. Marques, A. Khan, M. da Graca Miguel and B. Lindman, J. Phys. Chem., 1993, 97, 4729 CrossRef CAS.
- F. M. Menger, W. H. Binder and J. S. Keiper, Langmuir, 1997, 13, 3247 CrossRef CAS.
- J. Hao and H. Hoffmann, Curr. Opin. Colloid Interface Sci., 2004, 9, 279 CrossRef CAS PubMed.
- M. Rosa, M. Rosa Infante, G. Miguel Mda and B. Lindman, Langmuir, 2006, 22, 5588 CrossRef CAS PubMed.
- C. Bize, J.-C. Garrigues, M. Blanzat, I. Rico-Lattes, O. Bistri, B. Colasson and O. Reinaud, Chem. Commun., 2010, 46, 586 RSC.
- E. Soussan, S. Cassel, M. Blanzat and I. Rico-Lattes, Angew. Chem., Int. Ed., 2009, 48, 274 CrossRef CAS PubMed.
- E. Soussan, C. Mille, M. Blanzat, P. Bordat and I. Rico-Lattes, Langmuir, 2008, 24, 2326 CrossRef CAS PubMed.
- D. Vivares, E. Soussan, M. Blanzat and I. Rico-Lattes, Langmuir, 2008, 24, 9260 CrossRef CAS PubMed.
- A. Boudier, E. Soussan, P. Castagnos, G. Beaune, H. Belkhelfa, C. Menager, V. Cabuil, L. Haddioui, C. Roques, I. Rico-Lattes and M. Blanzat, Int. J. Pharm., 2011, 403, 230 CrossRef CAS PubMed.
- M. Blanzat, E. Perez, I. Rico-Lattes, D. Prome, J. C. Prome and A. Lattes, Langmuir, 1999, 15, 6163 CrossRef CAS.
- C. Mauroy, P. Castagnos, M.-C. Blache, J. Teissié, I. Rico-Lattes, M.-P. Rols and M. Blanzat, Chem. Commun., 2012, 48, 6648 RSC.
- S. Consola, M. Blanzat, E. Perez, J.-C. Garrigues, P. Bordat and I. Rico-Lattes, Chem.–Eur. J., 2007, 13, 3039 CrossRef CAS PubMed.
- C. Bize, J.-C. Garrigues, J.-P. Corbet, I. Rico-Lattes and M. Blanzat, ChemPhysChem, 2013, 14, 1126 CrossRef CAS PubMed.
- M. P. Siqueira-Moura, F. L. Primo, A. P. Peti and A. C. Tedesco, Pharmazie, 2010, 65, 9 CAS.
- S. M. T. Nunes, F. S. Sguilla and A. C. Tedesco, Braz. J. Med. Biol. Res., 2004, 37, 273 CrossRef CAS PubMed.
- M. N. Sibata, A. C. Tedesco and J. M. Marchetti, Eur. J. Pharm. Sci., 2004, 23, 131 CrossRef CAS PubMed.
- H. An, M. R. Nussio, M. G. Huson, N. H. Voelcker and J. G. Shapter, Biophys. J., 2010, 99, 834 CrossRef CAS PubMed.
- F. Denizot and R. Lang, J. Immunol. Methods, 1986, 89, 271 CrossRef CAS.
- A. Sparsa, S. Bellaton, T. Naves, M. O. Jauberteau, J. M. Bonnetblanc, V. Sol, M. Verdier and M. H. Ratinaud, Oncol. Rep., 2013, 29, 1196 CAS.
- P. Juzenas, A. Juzeniene, S. Stakland, V. Iani and J. Moan, Biochem. Biophys. Res. Commun., 2002, 297, 468 CrossRef CAS.
- B. Zhao, J. J. Yin, P. J. Bilski, C. F. Chignell, J. E. Roberts and Y. Y. He, Toxicol. Appl. Pharmacol., 2009, 241, 163 CrossRef CAS PubMed.
- E. C. C. Tapajós, J. P. Longo, A. R. Simioni, Z. G. M. Lacava, M. F. M. A. Santos, P. C. Morais, A. C. Tedesco and R. B. Azevedo, Oral Oncol., 2008, 44, 1073 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04876h |
| This journal is © The Royal Society of Chemistry 2014 |