Open Access Article
Open Access ArticleAn efficient synthesis of highly substituted indanones and chalcones promoted by superacid†
Amrita
Das
,
Alavala Gopi
Krishna Reddy
,
Jonnada
Krishna
and
Gedu
Satyanarayana
*
Indian Institute of Technology (IIT) Hyderabad, Ordnance Factory Estate Campus, Yeddumailaram – 502 205, Medak District, Andhra Pradesh, India. E-mail: gvsatya@iith.ac.in; Fax: +91(40) 2301 6032
First published on 9th June 2014
Abstract
A superacid promoted one-pot process for the efficient synthesis of indanones is presented. This process enabled the formation of a dual C–C bond between aryl isopropyl ketones and benzaldehydes. Interestingly, when the reaction was performed between acetophenones and benzaldehydes, it was impeded just after the aldol condensation and resulted in the corresponding chalcones.
Organic synthesis in a one-pot procedure is an indispensible technique due to its advantage of constructing more than one bond without the need to isolate the intermediate species. Therefore, those techniques that enable the formation of C–C bonds in a single step, particularly, for the synthesis of carbocyclic compounds are of significant importance. Because many such carbocyclic systems are present as core structure in many natural products of biological relevance. In this regard, among many classical C–C bond forming reactions, Friedel–Crafts reaction is treated as one of the best method for either alkylation or acylation discovered by Friedel and Crafts in 1877.1 Remarkably, in past few decades this reaction has been extensively applied in the field of organic synthesis under Brønsted/Lewis acidic conditions.2–4 Significantly, the Friedel–Crafts cyclization became an useful method for the synthesis of cyclic systems via single or multiple C–C bonds formation.5
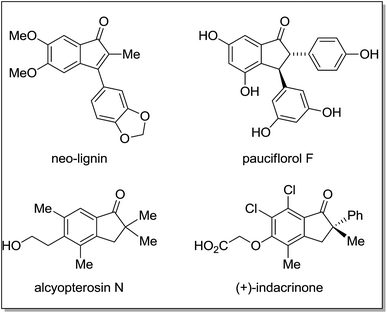
Notably, the superelectrophiles (more reactive intermediate species) concept was introduced by Olah et al.,6 which has been employed to build ring systems efficiently.3b As a part of our ongoing research interests on domino/sequential domino one-pot transformations,7 recently, we have reported the synthesis of indanones using simple cinnamate esters via dual C–C bond formation promoted by superacid.8 Also, very recently, we have developed mild method for the controlled formation of β-diaryl esters without the subsequent intramolecular acylation to give the indanones, via Friedel–Crafts Michael addition on cinnamate esters as key step for the synthesis of chromans.9 Indanones are ubiquitous systems that are present in many natural products, which show good range of biological activities as well as in a variety of drug candidates. Representative examples of such compounds include neo-lignin,10 pauciflorol F,11 alcyopterosin N,12 and indacrinone13 (Fig. 1).
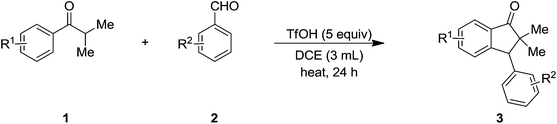
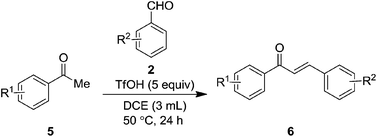
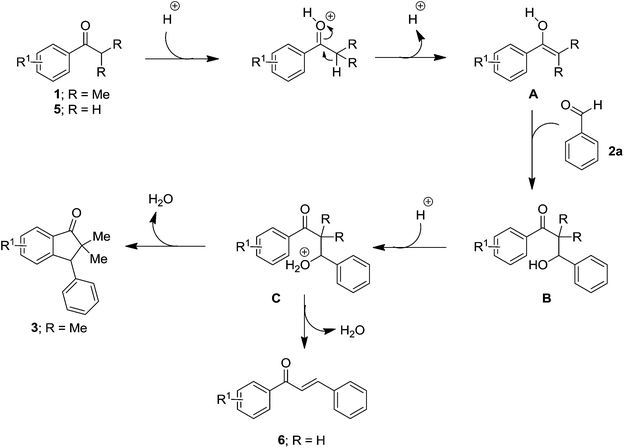
Because of the importance of indanone core, various acid mediated approaches have been reported on their synthesis.14 With this background, we envisaged that it would be feasible to generate enol selectively from aryl alkyl ketone under acidic reaction conditions. Thus the so formed enol of the ketone would act as a nucleophile and attack on the electrophilic aldehyde group in intermolecular fashion to give the β-hydroxy ketone intermediate which in turn is liable for subsequent intramolecular Friedel–Crafts alkylation to furnish the target indanones. Though, it can be realized that the intramolecular Friedel–Crafts alkylation will not be much favourable with an aromatic ring directly connected to a deactivating group (carbonyl), the idea behind this aim is based on the use of heating conditions in the presence of acid that may overcome such hurdles. Herein, we present an efficient one-pot method for the synthesis of highly substituted indanones via dual C–C bond formation promoted by superacid (triflic acid). On the other hand, we have noticed that the reaction between the acetophenones and benzaldehydes, impeded after aldol condensation and gave the corresponding chalcones as the end products.
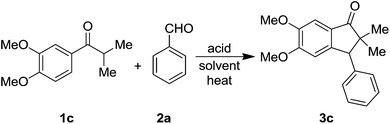
The required aryl isopropyl ketones for this study, were synthesized from the corresponding benzaldehydes using standard isopropyl Grignard addition and oxidation protocol (see, ESI†). To find out the best optimized reaction conditions, the ketone 1c was chosen as model and reacted with the benzaldehyde 2a under different reaction conditions in the presence of acid as promoting agent and the results are summarized in Table 1. Thus, the reactions of 1c with TFA either as reagent or as the reaction medium at 50 °C were not clean (Table 1, entries 1 & 2). On the other hand, treatment of 1c with superacid (triflic acid) in DCE at ambient temperature, furnished the product 3c, albeit in poor yield (30%) along with the recovery of the starting material 1c (Table 1, entry 4). However, when benzene was used as the solvent, the reaction was not clean (Table 1, entry 6). Interestingly, the reaction in hot CHCl3, improved the product 3c yield (50%, Table 1, entry 7). Gratifyingly, treatment of 1c in DCE at 50 °C, was found to be the best and furnished 3c as an exclusive product in good yield (85%, Table 1 entry 8). Use of concentrated H2SO4 also proved to be good and gave the product 3c in 70% yield (Table 1, entry 9). On the other hand, the reaction with p-TSA, led to the total recovery of starting material 1c (Table 1, entry 10). On the other hand, use of other Lewis acid (FeCl3), led to unclear reaction mixtures (Table 1, entry 11). Also the use of Lewis acid AlCl3 at 50 °C resulted into the product 3c in 61% yield (Table 1, entry 12).
| Entry | Acid (equiv.) | Solvent (mL) | Temp. (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yields of the pure products. b yield calculated based on the recovery of starting material. | |||||
| 1 | TFA (5) | DCE (2) | 50 | 12 | — |
| 2 | TFA | TFA (2) | 50 | 12 | — |
| 3 | TfOH (3) | DCE (2) | r.t. | 24 | 10 |
| 4 | TfOH (5) | DCE (2) | r.t. | 24 | 30 |
| 5b | TfOH (3) | DCE (2) | 50 | 36 | 57 |
| 6 | TfOH (5) | Benzene (2) | r.t. | 24 | — |
| 7 | TfOH (5) | CHCl3 (2) | 50 | 24 | 50 |
| 8 | TfOH (5) | DCE (2) | 50 | 24 | 85 |
| 9 | H2SO4 (5) | DCE (2) | 50 | 16 | 60 |
| 10 | p-TSA (3) | DCE (2) | 50 | 16 | — |
| 11 | FeCl3 (3) | DCE (2) | 50 | 16 | — |
| 12b | AlCl3 (3) | DCE (2) | 50 | 36 | 61 |
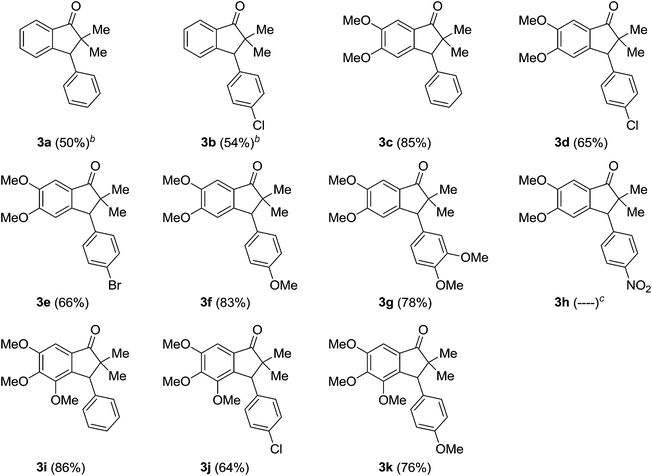
Among all screened reaction conditions, the entry 8 of Table 1 turned out to be the best with respect to the yield of the product 3c. Therefore, these conditions were applied to the other systems 1a–1d to check the scope and limitations of the method. Gratifyingly, it was proved to be amenable and furnished the corresponding indanones 3a–3j with dense functionality on either of the aromatic rings, in good yields as shown in Table 2. It is worth mentioning that the reaction was smooth with electron rich aromatic ring of the ketones 1b–1d. Whereas, in case of simple aromatic ketones 1a the reaction was found to be slow, as anticipated reaction rate depends on the electron rich nature of the aromatic ring. However, the reaction was successful by raising temperature from 50 °C to 80 °C, albeit in moderate yields of the products 3a and 3b (Table 2). While, further increasing the triflic acid amount (10 equivalents), led to the unclear reaction mixture. In general, the reaction was smooth for benzaldehydes 2 with simple to electron rich aromatic rings except 3,4,5-trimethoxybenzaldehyde 2g. In case of 3,4,5-trimethoxybenzaldehyde 2g, simple mono demethylation was observed from a para-methoxy group to the aldehyde group. The reaction was not clean with electron deficient para-nitrobenzaldehyde 2h, where, neither the product nor the corresponding starting material was isolated.
| a One-pot reaction conditions for the formation of indanones 3: ketones 1 (0.25 mmol), aldehydes 2 (0.50 mmol, 2 equiv.), TfOH (1.25 mmol, 5 equiv.) and DCE (1.5 mL) at 80 °C for 48 h for the formation of indanones 3a & 3b and at 50 °C for 24 h for other indanones 3c–3k formation. Yields in the parentheses are isolated yields of chromatographically pure products. b Yields based on the recovery of the starting material 1a. c The reaction furnished neither the product nor the recovery of the starting material. |
|---|
|
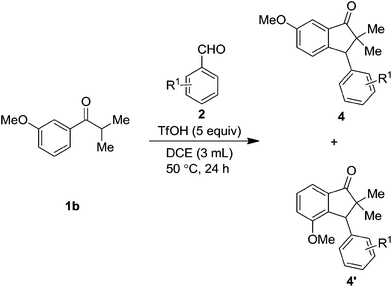
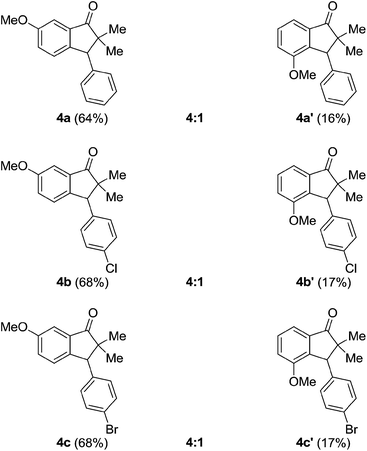
While, the reaction with 3-anisyl isopropyl ketone 1b furnished the regioisomeric mixture of indanones 4 & 4′ in almost 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios, in which, as expected, the major isomer was the one where cyclization occurred at para-position to the methoxy group and the results are as summarized in the Table 3.
1 ratios, in which, as expected, the major isomer was the one where cyclization occurred at para-position to the methoxy group and the results are as summarized in the Table 3.
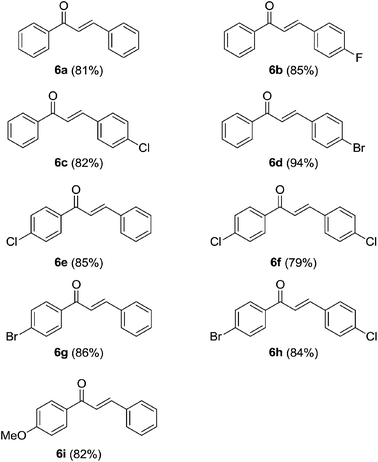
To further check the scope and generality of the method, we have attempted the reaction between acetophenones 5 and benzaldehydes 2 as well. Surprisingly, the reaction was impeded after the aldol condensation without subsequent cyclization (Table 4). This may be due to thermodynamic stability of enone systems. Moreover, to check the generality of the process, we have explored the reaction between different acetophenones 5 and benzaldehydes 2. Gratifyingly, the reaction was found to be quite successful and gave the corresponding chalcones 6 in very good to excellent yields as shown in Table 4.
| a Reaction conditions for the formation of chalcones 6: ketones 5 (0.50 mmol), aldehydes 2 (1.0 mmol, 2 equiv.), TfOH (2.5 mmol, 5 equiv.) and DCE (1.5 mL) at 50 °C for 24 h for the formation of chalcones 6a–6i. Yields in the parentheses are isolated yields of chromatographically pure products. |
|---|
|
The possible reaction mechanism for the formation of indanones 3 and chalcones 6 is outlined in Scheme 1. Initially, the acid can activate ketone through protonation to the carbonyl oxygen and yields the corresponding enol A. Nucleophilic attack of the enol A to the electrophilic aldehyde carbon furnishes the β-hydroxy ketone intermediate B. Since the β-hydroxy ketone intermediate B can be liable for intramolecular Friedel–Crafts alkylation in the presence of acid, it triggers to the cyclization through the intermediate C and generates the final indanone product 3. Similarly, in case of acetophenones, it yields the corresponding β-hydroxy ketone intermediate B. However, because of the availability of β-hydrogen for hydroxyl group it prefers dehydration than cyclization and furnishes the chalcone 6 products.
Conclusions
In summary, we have developed an efficient one-pot method for the synthesis of highly substituted indanones via dual C–C bond formation promoted by superacid. Significantly, these indanone systems are ubiquitous units that are present in drugs and many biologically active natural products. Interestingly, when acetophenones were treated with benzaldehydes in the presence of super acid, the reaction was impeded after aldol condensation and furnished the chalcones. Further, applications of this method to different structurally important carbocyclic compounds are under progress.Acknowledgements
Financial support by the Council of Scientific and Industrial Research [(CSIR), 02(0018)/11/EMR-II], New Delhi, is gratefully acknowledged. A. G. K. R. and J. K. thank CSIR, New Delhi, for the award of research fellowship.Notes and references
- C. Friedel and J. M. Crafts, Compt. Rend., 1877, 84, 1450 Search PubMed.
- For reviews, see: (a) S. Kobayashi, M. Sugiura, H. Kitagawa and W. W.-L. Lam, Chem. Rev., 2002, 102, 2227 CrossRef CAS PubMed; (b) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (c) J. M. Sartori and R. Maggi, Chem. Rev., 2011, 111, 181 CrossRef PubMed; (d) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6, 1–24 Search PubMed; (e) M. Shi, J.-M. Lu, Y. Wei and L.-X. Shao, Acc. Chem. Res., 2012, 45, 641 CrossRef CAS PubMed.
- (a) P. H. Gore and G. A. Olah, in Friedel–Crafts and Related Reactions, John Wiley and Sons, London, 1964, Part 1 vol. III, p. 1 Search PubMed; (b) G. A. Olah and D. A. Klumpp, Superelectrophiles and Their Chemistry, Wiley, New York, 2008 Search PubMed.
- (a) K. K. S. Sai, M. J. Tokarz, A. P. Malunchuk, C. Zheng, T. M. Gilbert and D. A. Klumpp, J. Am. Chem. Soc., 2008, 130, 14388 CrossRef CAS PubMed; (b) Y. Zhang, M. R. Sheets, E. K. Raja, K. N. Boblak and D. A. Klumpp, J. Am. Chem. Soc., 2011, 133, 8467 CrossRef CAS PubMed; (c) D. A. Evans and K. R. Fandrick, Org. Lett., 2006, 8, 2249 CrossRef CAS PubMed; (d) M. D. Rose, M. P. Cassidy, P. Rashatasakhon and A. Padwa, J. Org. Chem., 2007, 72, 538 CrossRef CAS PubMed; (e) Y.-C. Wu, L. Liu, Y.-L. Liu, D. Wang and Y.-J. Chen, J. Org. Chem., 2007, 72, 9383 CrossRef CAS PubMed.
- (a) T. Suzuki, T. Ohwada and K. Shudo, J. Am. Chem. Soc., 1997, 119, 6774 CrossRef CAS; (b) T. Ohwada, T. Suzuki and K. Shudo, J. Am. Chem. Soc., 1998, 120, 4629 CrossRef CAS; (c) H. Kurouchi, H. Sugimoto, Y. Otani and T. Ohwada, J. Am. Chem. Soc., 2010, 132, 807 CrossRef CAS PubMed; (d) H. M. Colquhoun, D. F. Lewis and D. J. Williams, Org. Lett., 2001, 3, 2337 CrossRef CAS PubMed; (e) E. Fillion and D. Fishlock, Org. Lett., 2003, 5, 4653 CrossRef CAS PubMed; (f) Q. Wang and A. Padwa, Org. Lett., 2006, 8, 601 CrossRef CAS PubMed; (g) S. Chassaing, M. Kumarraja, P. Pale and J. Sommer, Org. Lett., 2007, 9, 3889 CrossRef CAS PubMed; (h) A. Saito, M. Umakoshi, N. Yagyu and Y. Hanzawa, Org. Lett., 2008, 10, 1783 CrossRef CAS PubMed; (i) S. Tang, Y. Xu, J. He, Y. He, J. Zheng, X. Pan and X. She, Org. Lett., 2008, 10, 1855 CrossRef CAS PubMed; (j) C. O. Kangani and B. W. Day, Org. Lett., 2008, 10, 2645 CrossRef CAS PubMed; (k) K. Kim and I. Kim, Org. Lett., 2010, 12, 5314 CrossRef CAS PubMed; (l) R. K. Chinnagolla and M. Jeganmohan, Org. Lett., 2012, 14, 5246 CrossRef CAS PubMed; (m) D. Eom, S. Park, Y. Park, T. Ryu and P. H. Lee, Org. Lett., 2012, 14, 5392 CrossRef CAS PubMed; (n) D. A. Klumpp, D. N. Baek, G. K. S. Prakash and G. A. Olah, J. Org. Chem., 1997, 62, 6666 CrossRef CAS; (o) R. Rendy, Y. Zhang, A. McElrea, A. Gomez and D. A. Klumpp, J. Org. Chem., 2004, 69, 2340 CrossRef CAS PubMed; (p) S. S. Bhar and M. M. V. Ramana, J. Org. Chem., 2004, 69, 8935 CrossRef CAS PubMed; (q) G. B. Womack, J. G. Angeles, V. E. Fanelli and C. A. Heyer, J. Org. Chem., 2007, 72, 7046 CrossRef CAS PubMed; (r) K. K. S. Sai, P. M. Esteves, E. T. D. Penha and D. A. Klumpp, J. Org. Chem., 2008, 73, 6506 CrossRef CAS PubMed; (s) G. K. S. Prakash, F. Paknia, H. Vaghoo, G. Rasul, T. Mathew and G. A. Olah, J. Org. Chem., 2010, 75, 2219 CrossRef CAS PubMed; (t) E. K. Raja, D. J. DeSchepper, S. O. N. Lill and D. A. Klumpp, J. Org. Chem., 2012, 77, 5788 CrossRef CAS PubMed; (u) Y. L. Choi, B. T. Kim and J.-N. Heo, J. Org. Chem., 2012, 77, 8762 CrossRef CAS PubMed; (v) S. J. Mahoney, D. T. Moon, J. Hollinger and E. Fillion, Tetrahedron Lett., 2009, 50, 4706 CrossRef CAS PubMed; (w) H. Aikawa, S. Tago, K. Umetsu, N. Haginiwa and N. Asao, Tetrahedron, 2009, 65, 1774 CrossRef CAS PubMed.
- G. A. Olah, A. Germain, H. C. Lin and D. A. Forsyth, J. Am. Chem. Soc., 1975, 97, 2928 CrossRef CAS.
- (a) A. G. K. Reddy and G. Satyanarayana, Tetrahedron, 2012, 68, 8003 CrossRef CAS PubMed; (b) L. Mahendar, J. Krishna, A. G. K. Reddy, B. V. Ramulu and G. Satyanarayana, Org. Lett., 2012, 14, 628 CrossRef CAS PubMed; (c) A. G. K. Reddy, J. Krishna and G. Satyanarayana, Tetrahedron Lett., 2012, 53, 5635 CrossRef PubMed; (d) A. G. K. Reddy, J. Krishna and G. Satyanarayana, Tetrahedron, 2013, 69, 10098 CrossRef CAS PubMed; (e) L. Mahendar and G. Satyanarayana, J. Org. Chem., 2014, 79, 2059 CrossRef CAS PubMed; (f) J. Krishna, A. G. K. Reddy and G. Satyanarayana, Synlett, 2013, 24, 967 CrossRef CAS PubMed; (g) J. Krishna, A. G. K. Reddy and G. Satyanarayana, Tetrahedron Lett., 2014, 55, 861 CrossRef CAS PubMed.
- B. V. Ramulu, A. G. K. Reddy and G. Satyanarayana, Synlett, 2013, 24, 868 CrossRef CAS PubMed.
- B. Suchand, J. Krishna, K. Mritunjoy and G. Satyanarayana, RSC Adv., 2014, 4, 13941 RSC.
- (a) L. M. X. Lopes, M. Yoshida and O. R. Gottlieb, Phytochemistry, 1984, 23, 2021 CrossRef CAS; (b) D. C. Harrowven, N. A. Newman and C. A. Knight, Tetrahedron Lett., 1998, 39, 6757 CrossRef.
- T. Ito, T. Tanaka, M. Iinuma, K.-i. Nakaya, Y. Takahashi, R. Sawa, J. Murata and D. Darnaedi, J. Nat. Prod., 2004, 67, 932 CrossRef CAS PubMed.
- J. A. Palermo, M. F. Rodriguez Brasco, C. Spagnuolo and A. M. Seldes, J. Org. Chem., 2000, 65, 4482 CrossRef CAS.
- (a) U.-H. Dolling, P. Davis and E. J. J. Grabowski, J. Am. Chem. Soc., 1984, 106, 446 CrossRef CAS; (b) S. J. deSolms, O. W. Woltersdorf Jr and E. J. Cragoe Jr, J. Med. Chem., 1978, 21, 437 CrossRef CAS.
- (a) D.-M. Cui, C. Zhang, M. Kawamura and S. Shimada, Tetrahedron Lett., 2004, 45, 1741 CrossRef CAS PubMed; (b) E. Fillion, D. Fishlock, A. Wilsily and J. M. Goll, J. Org. Chem., 2005, 70, 1316 CrossRef CAS PubMed; (c) M. B. Floyd and G. A. Allen Jr, J. Org. Chem., 1970, 35, 2647 CrossRef CAS; (d) A. V. Vasilyev, S. Walspurger, P. Pale and J. Sommer, Tetrahedron Lett., 2004, 45, 3379 CrossRef CAS PubMed; (e) N. J. Lawrence, E. M. S. Armitage, B. Greedy, D. Cook, S. Ducki and A. T. McGown, Tetrahedron Lett., 2006, 47, 1637 CrossRef CAS PubMed; (f) W. Yin, Y. Ma, J. Xu and Y. Zhao, J. Org. Chem., 2006, 71, 4312 CrossRef CAS PubMed; (g) J. Petrignet, T. Roisnel and R. Grée, Chem.–Eur. J., 2007, 13, 7374 CrossRef CAS PubMed; (h) L. Liu, L. Wei, Y. Lu and J. Zhang, Chem.–Eur. J., 2010, 16, 11813 CrossRef CAS PubMed; (i) P. Dubé and F. D. Toste, J. Am. Chem. Soc., 2006, 128, 12062 CrossRef PubMed; (j) D. H. Dethe and G. Murhade, Org. Lett., 2013, 15, 429 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04763j |
| This journal is © The Royal Society of Chemistry 2014 |