Facile synthesis and enhanced photocatalytic activity of Sm(OH)3 nanorods†
Wang Dan,
Huang Jianfeng*,
Yin Lixiong,
Ouyang Haibo,
Li Jiayin and
Wu Jianpeng
School of Materials Science & Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China. E-mail: huangjfsust@126.com; Fax: +86 029 86168802; Tel: +86 029 86168802
First published on 18th July 2014
Abstract
Samarium hydroxide (Sm(OH)3) nanorods with enhanced photocatalytic activity to degrade RhB were prepared by a facile precipitation method. The phase composition, morphology and optical properties of the as-prepared sample were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy and UV-vis diffuse reflectance spectroscopy. The results show that the as-prepared Sm(OH)3 nanocrystallites are hexagonal phase with a rod-like microstructure, and exhibit a strong absorption ability of UV light. Moreover, the low temperature precipitation synthesis introduced an amorphous layer on the Sm(OH)3 nanorods, which was confirmed to have a positive impact on improving the photocatalytic activity of Sm(OH)3 nanorods.
1. Introduction
Lanthanide compounds have attracted considerable interest over the past several years because of their novel optical,1 electronic2 and chemical3 properties arising from their 4f electrons. Lanthanide hydroxides are a typical kind of functional lanthanide compounds that are generating increasing enthusiasm among researchers for the synthesis of one-dimensional (1D) nanostructures to investigate their interesting properties.4,5 Numerous synthesis techniques have been developed to prepare 1D nano/microsized inorganic materials such as the hydrothermal technique,6 sol–gel route7 and chemical conversion method.8 Moreover, the hydrothermal process9 and the homogeneous precipitation method10 have been widely used to prepare 1D lanthanide hydroxides, due to their facile preparation, high efficiency, and low cost. Sm(OH)3 is a promising lanthanide hydroxide material, and in our previous research, hexagonal prism-like Sm(OH)3 nanocrystallites were prepared for the photocatalytic degradation of rhodamine B (RhB) by the hydrothermal process.11 To deeply investigate the properties of Sm(OH)3 nanocrystallites, facile and controllable synthesis methods are critical.In the present work, a low-temperature precipitation method is proposed to prepare Sm(OH)3 nanorods efficiently. First, the phase composition and microstructure of the as-prepared sample were investigated. Second, the enhanced photocatalytic activity of the sample was successfully achieved. Finally, the reason for the enhanced photocatalytic activity of the as-prepared sample was analyzed.
2. Experimental
Sm(NO3)3·6H2O and diethylenetriamine (DETA) were of analytical reagent (A.R.) grade and used without further purification. First, 1.5 mmol Sm(NO3)3·6H2O was dissolved in 60 mL distilled water, then 0.28 mL DETA was added dropwise with magnetic stirring to form the precursor solution. The precursor solution was transferred to a 100 mL flask and thermally aged at 60 °C in a water-bath for 2 h after stirring for 1 h. Subsequently, the product was centrifuged and washed with distilled water and anhydrous ethanol several times, before being finally dried in a vacuum drying oven at 60 °C for 3 h. The weight of the dried product was measured through a precision balance with a sensitivity of ±0.1 mg. The reaction yield was calculated to be 84.85% by eqn (1), which suggests the raw materials were fully utilized in the reaction.| Reaction yield (%) = (m/m0) × 100% | (1) |
The crystalline microstructure of the as-prepared powder was characterized by powder X-ray diffraction (XRD, Rigaku D/max-2000) with Cu Kα radiation (λ = 0.15406 nm) at 40 kV and 40 mA in the 2θ range of 10°–60°. The morphology of the sample was observed by field emission scanning electron microscopy (FE-SEM, Hitachi S-4800, Acceleration voltage: 3 kV). High-resolution transmission electron microscopy (HRTEM operated at 200 kV) was taken by field emission transmission electron microscopy (FE-TEM, American FEI Tecnai G2 F 20 S-TWIN). The UV-vis diffuse reflectance spectrum of the sample was measured by a Shimadzu UV-2450 UV-vis spectrophotometer.
Photocatalytic activities of the prepared Sm(OH)3 nanorods were evaluated by photocatalytic degradation of 5 mg L−1 Rhodamine B, methyl orange, and neutral red aqueous solution. The photocatalytic activity tests were carried out using a BL-GHX-V photocatalytic reactor (Xi'an, BILOBN, Co. Ltd) with a 500 W mercury lamp as the UV light source. The loading amount of catalysts was 1.0 g L−1. Before illumination, the suspensions of dyes with catalysts were magnetically stirred in the dark for 30 min, after being dispersed in an ultrasonic bath for 5 min, to ensure the establishment of an adsorption–desorption equilibrium between the catalysts and dyes. Then, the solution was exposed to a 500 W mercury lamp under magnetic stirring. By prolonging the irradiation time, 6 mL of the solution was collected with centrifugation at 5 min intervals. The concentrations of the remnant dyes in the collected solution were monitored by UV-vis spectroscopy (Unico UV-2600) at 553 nm.11 In the photocatalytic reaction process, the degradation efficiency of dyes was calculated by eqn (2):
| Degradation efficiency (%) = (1 − Ct/C0) × 100% | (2) |
3. Results and discussion
3.1. Phase analysis
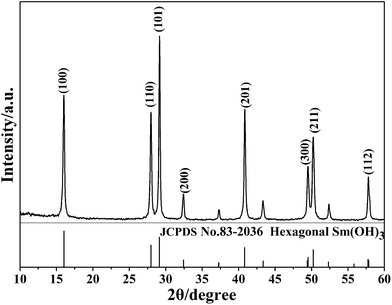
The XRD pattern of the sample prepared by a facile precipitation method is shown in Fig. 1. The XRD peaks of the as-prepared sample can be finely indexed to the hexagonal Sm(OH)3 (JCPDS no. 83-2036). No characteristic peaks of impurities can be detected, indicating that the pure phase of Sm(OH)3 was achieved under the current synthetic conditions.3.2. Morphological analysis
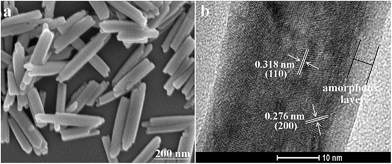
Fig. 2(a) shows the SEM image of the Sm(OH)3 nanocrystallites prepared by the facile precipitation method. It can be clearly observed that the microstructure of the prepared sample is rod-like and the average length of the nanorods is about 300 nm. Fig. 2(b) exhibits the high magnification TEM image of an individual Sm(OH)3 nanorod. The lattice spacing of d(200) = 0.276 nm and d(110) = 0.318 nm are clearly observed from Fig. 2(b), which means that the prepared Sm(OH)3 nanorods are polycrystal. The Sm(OH)3 nanorods may be composed of numerous Sm(OH)3 crystalline subunits with various orientations. Moreover, the high magnification TEM image of an individual Sm(OH)3 nanorod shows that the prepared Sm(OH)3 nanorods are wrapped in a layer of amorphous particles. This layer of amorphous particles was only found on the Sm(OH)3 nanorods prepared by the precipitation method, in contrast with the high-resolution TEM image (Fig. S1b†) of the well-crystallized Sm(OH)3 nanorods prepared by the hydrothermal method.3.3. Optical and photocatalytic properties
UV-vis diffuse spectroscopy was used to characterize the optical absorbance of the as-prepared Sm(OH)3 nanorods. Fig. 3 presents the direct band-gap energy estimated from a plot of (αhν)2 vs. the photo energy (hν), according to the K–M model. The optical band-gap of the Sm(OH)3 nanorods is calculated as 4.25 eV. Moreover, the UV-vis diffuse spectroscopy of the as-prepared Sm(OH)3 nanorods is shown in Fig. 3 (inset), in which Sm(OH)3 nanorods exhibit a strong band edge absorption in the region between 200–300 nm. By combing the good ultraviolet absorbing properties and the unique properties of lanthanide compounds,1–3 the photocatalytic activity of Sm(OH)3 nanorods was investigated.12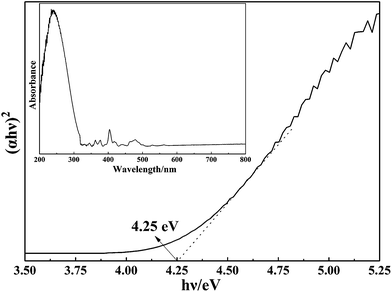 | ||
| Fig. 3 The relationship between (αhν)2 and photon energy (inset: UV-vis diffuse reflectance spectrum of Sm(OH)3 nanorods). | ||
The degradation of rhodamine B (RhB), methyl orange (MO), and neutral red (NR) were used to evaluate the photocatalytic activity of the as-prepared Sm(OH)3 nanorods, and the corresponding photocatalytic results are shown in Fig. 4(a). The photocatalytic results show that Sm(OH)3 nanorods can mainly degrade RhB and Neutral red in only 30 min, with the degradation efficiency reaching 94.3% and 97.3%, respectively. This means that the prepared Sm(OH)3 nanorods have a good responsiveness to the cationic (RhB) and neutral (neutral red) dyes. However, the degradation of neutral red is also very quick without photocatalysts, which affects the proper evaluation of the as-prepared Sm(OH)3 nanorods. Therefore, the degradation of RhB was used to compare the photocatalytic activity of the as-prepared Sm(OH)3 nanorods with other products.
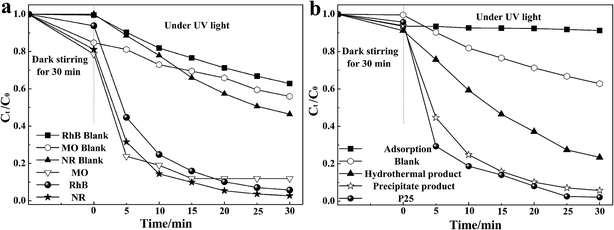 | ||
| Fig. 4 (a) Photocatalytic results of RhB, MO, and NR degraded by Sm(OH)3 nanorods, (b) photocatalytic results of RhB degraded by different kinds of photocatalysts. | ||
The photocatalytic results of RhB degraded by different kinds of photocatalysts are shown in Fig. 4(b). The adsorption test shows that the adsorption–desorption equilibrium between Sm(OH)3 nanorods and RhB was achieved after stirring in the dark for 30 min. The blank test demonstrates that the degradation of RhB is very slow without photocatalysts. When the prepared Sm(OH)3 nanorods were used as photocatalysts, the RhB absorption peak decreased quickly (Fig. S2†) as the irradiation time was prolonged. The photocatalytic results show that the as-prepared Sm(OH)3 nanorods can mostly degrade RhB in only 30 min, with the degradation efficiency reaching 94.3%; whereas, the degradation efficiency of the fully crystallized Sm(OH)3 nanocrystallites prepared by the hydrothermal process only reaches 76.5%. These results show that the Sm(OH)3 nanorods prepared by the precipitation method exhibit an enhanced photocatalytic activity to degrade RhB.
The microstructures and sizes of the precipitation and hydrothermal products are similar (Fig. S3†). The better photocatalytic activity of the Sm(OH)3 nanorods prepared by the precipitation method may be due to the amorphous particles wrapped on the Sm(OH)3 nanorods. The functionalized surface seems to enhance the adsorption amount of dissolved oxygen and RhB molecules. This makes the lifetime of the photogenerated holes longer, and these can then participate in the degradation process by the formation of reactive radicals or by the direct oxidation of the pollutant, thereby promoting the photocatalytic reaction efficiency.13 Moreover, the degradation efficiency of RhB could reach 97.9% when the well-known commercial photocatalyst P25 was used as the catalyst. This means that the photocatalytic activity of the prepared Sm(OH)3 nanorods still needs to be improved by further exploration studies. The small gap between the prepared Sm(OH)3 nanorods and the well-known commercial photocatalyst P25 suggests that the improved Sm(OH)3 nanocrystallites have the potential to be used as photocatalysts or as co-catalyst materials in the future.
4. Conclusion
In summary, pure hexagonal phase Sm(OH)3 nanorods have been successfully prepared by a facile and efficient precipitation method at 60 °C for 2 h using Sm(NO3)3·6H2O and DETA as raw materials. The prepared sample exhibits a high photocatalytic activity to degrade RhB with the degradation efficiency reaching 94.3% under UV irradiation for 30 min. The enhanced photocatalytic activity of Sm(OH)3 nanorods is attributed to the presence of an amorphous surface layer. It is expected that further research into the photocatalytic mechanisms of Sm(OH)3 nanocrystallites will be carried out in the future.Acknowledgements
The authors are grateful to National Key Technology R&D Program (no. 2013BAF09B02), International Science and Technology Cooperation Project Funding of Shaanxi Province (no. 2011 KW-11), Innovation Team Assistance Foundation of Shaanxi Province (2013KCT-06), Innovation Team Assistance Foundation of Shaanxi University of Science and Technology (no. TD09-05), and Graduate Innovation Fund of Shaanxi University of Science and Technology.References
- G. F. Wang, Q. Peng and Y. D. Li, Acc. Chem. Res., 2011, 44, 322–332 CrossRef CAS PubMed.
- I. Djerdj, G. Garnweitner, D. S. Su and N. Markus, J. Solid State Chem., 2007, 180, 2154–2165 CrossRef CAS PubMed.
- E. Van Der Kolk and P. Dorenbos, Chem. Mater., 2006, 18, 3458–3462 CrossRef CAS.
- J. S. Xie, Q. S. Wu, D. Zhang and Y. P. Ding, Cryst. Growth Des., 2009, 9, 3889–3897 CAS.
- Q. Y. Mu and Y. D. Wang, J. Alloys Compd., 2011, 509, 396–401 CrossRef CAS PubMed.
- Y. C. Zhu, T. Mei, Y. Wang and Y. T. Qian, J. Mater. Chem., 2011, 21, 11457–11463 RSC.
- Q. Kuang, Z. W. Lin, W. Lian, Z. Y. Jiang and Z. X. Xie, et al., J. Solid State Chem., 2007, 180, 1236–1242 CrossRef CAS PubMed.
- G. Jia, H. P. You, Y. H. Song, J. J. Jia and Y. H. Zheng, et al., Inorg. Chem., 2009, 48, 10193–10201 CrossRef CAS PubMed.
- X. Wang and Y. D. Li, Chem.–Eur. J., 2003, 9, 5627–5635 CrossRef CAS PubMed.
- N. Zhang, R. Yi, L. B. Zhou, G. H. Gao and R. G. Shi, et al., Mater. Chem. Phys., 2009, 114, 160–167 CrossRef CAS PubMed.
- D. Wang, J. F. Huang, L. X. Yin, L. Y. Cao and H. B. Ouyang, et al., Mater. Lett., 2014, 116, 393–395 CrossRef CAS PubMed.
- J. F. Huang, D. Wang, L. X. Yin, L. Y. Cao and H. B. Ouyang, et al., J. Alloys Compd., 2014, 612, 233–238 CrossRef CAS PubMed.
- M. Krivec, R. A. Segundo, J. L. Faria and A. M. T. Silva, et al., Appl. Catal., B, 2013, 140–141, 9–15 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04709e |
| This journal is © The Royal Society of Chemistry 2014 |