Enzyme-free selective determination of H2O2 and glucose using functionalized CuNP-modified graphite electrode in room temperature ionic liquid medium†
Rajendran Suresh Babu,
Pandurangan Prabhu and
Sangilimuthu Sriman Narayanan*
Department of Analytical Chemistry, School of Chemical Sciences, University of Madras, Guindy Campus, Chennai 600 025, Tamil Nadu, India. E-mail: sriman55@yahoo.com; Fax: +91-44-22352494; Tel: +91-44-22202717
First published on 11th August 2014
Abstract
A novel enzyme-free modified electrode constructed by electropolymerization of L-cysteine on a graphite electrode, followed by electrodeposition of copper nanoparticles using ionic liquid as a green electrolyte. The modified electrode exhibits an excellent electrocatalytic activity towards the oxidation of hydrogen peroxide (H2O2) and glucose at a reduced overpotential of 0 V and +0.35 V, respectively. The immobilization of copper nanoparticle encapsulated by polycysteine (PC) formed on the electrode was confirmed by Fourier transform infrared (FT-IR) spectroscopy, Raman spectra, X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) and atomic force microscopy (AFM) studies. The determination of H2O2 and glucose with the modified electrode shows the advantages of ease of preparation, high sensitivity, good selectivity and stability. The practical application of the modified electrode for selective detection of H2O2 and glucose has been evaluated by analyzing the real samples of stain remover solutions and human urine samples to determine H2O2 and glucose, respectively.
1. Introduction
The pursuit of enzyme-free H2O2- and glucose-sensing is an exciting and competitive area of research and technology. The development and fabrication of cost effective, simple, accurate, portable and rapid sensors for H2O2 and glucose is important in biology. Hydrogen peroxide plays a significant role in the chemical and pharmaceutical industries as an oxidizing, bleaching and sterilizing agent. It also acts as an oxidant in heterogeneous processes that create sulphuric acid and nitric acid in rain and in the atmosphere. At high concentrations, H2O2 causes irritation to the eyes and skin, thereby affecting human health.1 Furthermore, the detection of H2O2 is an important task in many biological, medical and clinical studies2,3 since H2O2 is consumed during the reactions of many oxidases, which in turn provide the basis for the construction of several peroxide biosensors. Among the various methods reported for the determination of H2O2, electroanalytical methods are generally preferred4–9 because of their simplicity, low detection limits, rapid response and relatively low cost.9 The detection of H2O2 at most kinds of bare electrodes requires a high overpotential, which in turn causes interference from many other electroactive and co-existing species. Biosensors that use peroxidase-modified electrodes have also been reported to provide an attractive method for H2O2 determination in recent years.10 Although biosensors provide sensitive determination of H2O2, drawbacks associated with their fabrication and long-term stability limit their application.Glucose is one among the biologically significant substrates that plays an essential role in biomedical, industrial and clinical applications. The importance of glucose in human metabolism is well known because the glucose levels determine the extent of complications in diseases such as diabetes and heart diseases, and in kidney, vision and nerve damage. Thus, there is always an increasing demand for the development of new methodologies for simple, rapid and reliable quantification of glucose. Despite immense advances in glucose sensing, there are still many challenges related to the achievement of simple and sensitive sensors possessing improved and highly desirable analytical characteristics for selective detection. Conventional techniques such as spectrophotometry are limited in identifying and quantifying glucose due to the lack of chromophoric and fluorophoric ligands in glucose.11 Amperometric glucose sensors are of two major types, i.e., enzymatic and nonenzymatic sensors, and they have received considerable interest and have been developed rapidly due to their advantages of high sensitivity and quick response.12 Since Clark reported the first enzyme electrode in 196213 and Updike constructed the first enzymatic biosensor to amperometrically detect glucose in 1967,14 a number of studies have focused on developing electrochemical enzymatic glucose sensors over the last four decades.15 As a result of the drawbacks of enzymatic sensor in applications to lab-on-a-chip and in vitro glucose assay, such as difficulties in miniaturization, instability of the enzyme, poor reproducibility and interference of oxygen,16 many researchers have considered electrochemical determination of glucose without using enzyme. In fact, an enzyme-free glucose sensor has been one of the ideas of many researchers.16,17
Several enzyme-free glucose and H2O2 sensors have been reported by using different electrode materials such as Pt, Pd, Au, Ag, Bi, Hg, Ni, Cu and some alloys such as Pt–Pd, Pt–Au, Au–Ru, etc. have been investigated.18–20 These have certain drawbacks such as low sensitivity, poor selectivity, and poisoning by adsorbed intermediates and chloride ions.21 Out of all these electrode materials, Cu and CuO-based electrode materials are shown to facilitate the inherent tendency for mediated electro oxidation of enzyme-free glucose and H2O2 determination. Moreover, CuO is inexpensive, more stable in air, good sensitivity, excellent selectivity and shows no observable self-poisoning effect in solutions,22 owing CuO based electrodes is very difficult to form complexation with Cl− ion since the electronegativity of O is higher than that of Cl− ion. Thus, the significance of the electrodes modified with the functionalized CuNP based materials for enzyme-free biosensors.
Numerous enzyme and enzyme-free sensors based on electrochemical techniques for the determination of H2O2 and glucose have been explored in our laboratory.23–25 In this report, we propose to fabricate the electropolymerization of L-cysteine and electrodeposition of Cu nanoparticles on paraffin wax-impregnated graphite electrode (PIGE) using ionic liquid as a green electrolyte. The surface morphology of polycysteine-functionalized copper nanoparticle (PCFCuNP)-modified electrode has been examined by using FTIR-ATR, Raman spectroscopy, XPS, TEM and AFM. The modified electrode was successfully employed for the detection of H2O2 and glucose in the alkaline medium using cyclic voltammetry (CV) and linear sweep voltammetric (LSV) techniques. The proposed modified electrode was successfully employed for the amperometric detection of H2O2 and glucose. To demonstrate the biological and industrial significance of the modified electrode, we examined the determination of H2O2 and glucose in real samples using stain remover solutions and urine samples, respectively.
2. Experimental
2.1 Reagents and equipment
L-Cysteine (L-Cys), epinephrine (EP), D-glucose, hydrogen peroxide, ascorbic acid (AA), dopamine (DA), uric acid (UA) were purchased from HiMedia Laboratories Pvt. Ltd. 1-Ethyl-3-methylimidazolium ethyl sulphate (purity 98.5%) was obtained from Alfa Aesar. Spectroscopic grade graphite rod (3 mm diameter) was used as received from Aldrich. Two different commercial stain remover solutions were purchased from a local market. All other chemicals were of analytical grade and doubly distilled water was used in the experiments.FT-IR spectra were recorded using a Perkin-Elmer RX 1 spectrometer. Raman spectra were recorded with Raman 11i system (Nanophoton Corp., Japan). XPS measurement for surface analysis was performed with monochromatic 300 W Al Kα X-ray radiation as the X-ray source for excitation (Model XM 1000, Omicron Nanotechnology, Germany). In this study, the morphology and size of PCFCuNP-modified electrode were measured on a AFM (XE 70 parks system) and TEM images were obtained using an Hitachi H7650 Microscope. Electrochemical experiments were performed with a CHI 660 B electrochemical analyzer (CH Instruments, USA). All experiments were performed in a conventional three-electrode cell at room temperature. A graphite electrode-modified with PCFCuNP was used as the working electrode. A platinum electrode was used as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. For AFM characterization, the PCFCuNP was modified on ITO electrode instead of graphite electrode. All electrochemical experiments were performed in 0.1 M NaOH solution.
2.2 Fabrication of PCFCuNP-modified electrode
Paraffin-impregnated graphite electrode (PIGE) was prepared as reported26 by heating spectroscopic grade graphite rods (0.3 cm circular diameter, 4.0 cm length) in molten wax with the application of suction for half an hour until air bubbles ceased to evolve from the rods. After re-establishing atmospheric pressure, the rods were removed before the paraffin solidified. The lower end of the electrode was polished with finest quality emery paper, and the final mirror finish for modification was obtained with velvette cloth. Mirror polish helps to minimize the residual current. The polished surface was then washed with methanol. The functionalized nickel nanoparticle was prepared as described earlier.27 The electrochemical deposition of PC was carried out from a room temperature ionic liquid as a green electrolyte containing 10 mM L-Cys monomer and 0.1 M 1-ethyl-3-methylimidazolium ethyl sulphate by cycling the potential between −0.6 and 2.0 V at 0.05 V s−1. A thin film made with 15 deposition cycles was used in all experiments. When removed from the solution, the PC-modified electrode was rinsed with distilled water to remove unbound materials from the electrode surface and then dried under atmospheric condition.CuNPs were deposited potentiostatically on the PC-modified electrode in a solution of 0.1 M 1-ethyl-3-methylimidazolium ethyl sulphate and 1 mM CuSO4 by using amperometric technique at preselected potentials. A constant potential of −1.2 V for 100 s was applied (with respect to SCE reference electrode) for CuNP deposition. The PCFCuNP-modified electrode was rinsed thoroughly with water and dried at room temperature. HRTEM and AFM were used to examine the surface morphology of the modified electrode and to characterize the shape, size, and density of the CuNPs.
3. Results and discussion
3.1 FTIR spectra, Raman spectra and XPS of PCFCuNP-modified electrode
The FTIR study of the PCFCuNP-modified electrode was investigated in the reflection mode. The FTIR spectra of PCFCuNP-modified electrode are shown in Fig. S1 (see ESI†). In the spectra, the most important band at 2553 cm−1 corresponds to the thiol (–SH) group in L-cysteine.27 In the case of PCFCuNP-modified electrode, the peak at 2553 cm−1 has disappeared (Fig. S1†). The disappearance of the peak is attributed to the cleavage of the S–H bond and the formation of the S–Cu bond, which is in confirmation with the XPS study.28 The peaks at 475 and 432 cm−1 confirm the formation of Cu–S bond.29 According to FTIR spectroscopic studies,29 PC can bind onto MNP through the strong sulfur–metal interaction. Based on the above results, it seems that only the thiol group is involved in the bonding with the Cu surface.The Raman spectrum of L-cysteine and PC-modified electrode shows absorption at 2565 cm−1, which indicates the presence of a free –SH group in L-Cys- and PC-modified electrode27 (curves not shown). The absence of a peak at this position for PCFCuNP-modified electrode confirms the absence of a free –SH group in the modified electrode. The absorption at 1579 cm−1 corresponds to NH3+ symmetrical deformation and is present in PC-modified electrode as well as PCFCuNP-modified electrode, indicating the presence of PC in both electrodes. The peak at 471 cm−1 confirms the formation of Cu–S bond (see ESI Fig. S2†), and these observations are consistent with earlier report.30
To prove that PC and CuNPs had been immobilized onto the surface of the electrode, XPS experiments were performed (see Fig. 1a). Two XPS bands of Cu appear at 932.3 and 952.1 eV, corresponding to the Cu (2p3/2) and Cu (2p1/2) signals, respectively (Fig. 1b), which demonstrates the immobilization of CuNP on the PC-modified electrode surface. Another two peaks are observed due to the existence of O impurity in the sample, which originated from surface contamination of CuNP. The S (2p3/2) signal appearing at 162.5 eV indicates the formation of S–Cu bond on the modified electrode (Fig. 1c).31,32 The above results demonstrate that CuNPs were chemically bound to the surface of the PC-modified electrode and confirm the deposition of CuNPs on the electrode surface. The above results were consistent with the previous reports.33,34
 | ||
| Fig. 1 XPS spectra of (a) survey spectra, (b) 2P3/2 and 2P1/2 peaks of Cu, (c) 2P3/2 peak of S of the PCFCuNP-modified electrode. | ||
3.2 AFM and TEM studies of PCFCuNP-modified electrode
The size and morphology of the modified electrode were investigated by AFM and HRTEM. The AFM topographic images of bare electrode showed the bare substrate consisting of a smooth surface morphology (see ESI Fig. S3†), whereas the PCFCuNP-modified electrode showed a rough surface morphology, obtained with homogenous deposition of CuNPs on modified electrode surface (Fig. 2). The roughness increased from the bare electrode to the modified electrode surface after electrodeposition of CuNPs, which clearly showed that the CuNPs uniformly electrodeposited on the electrode surface. The benefit of using 1-ethyl-3-methylimidazolium ethyl sulphate as an electrolyte is more rapid nucleation rate, higher conductivity and viscosity than in conventional solvents. The size and shape of the nanoparticles were also characterized and confirmed by HRTEM. The image represents the uniform deposition of CuNPs mainly on PC surface. This also indicates the formation of CuNPs with a spherical-like structure, and the size of each nanoparticle was found to be ∼20–30 nm, as shown in Fig. 3.3.3 Electrocatalytic oxidation of H2O2 at PCFCuNP-modified electrode
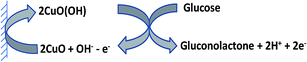
The electrocatalytic activity of PCFCuNP-modified electrode for H2O2 oxidation was explored in this study. The CV of the PCFCuNP-modified electrode in 0.1 M NaOH containing 2 mM H2O2 compared with that of bare electrode CV is shown in Fig. 4A (curves a and b). The electro-oxidation of H2O2 at the bare electrode required a large overpotential, and a poor anodic current was observed at +0.50 V as shown in Fig. 4A (curve b). The catalytic oxidation of H2O2 at the modified electrode can be clearly seen in Fig. 4A (curve d), where the peak current is markedly enhanced and the peak potential shifted negatively at 0 V. The oxidation current of CuNP was greatly increased due to the electrocatalytic oxidation of H2O2 at 0 V. The concentration dependence of the catalytic current for successive additions of H2O2 at the modified electrode resulted in a good linear relationship between the catalytic current and H2O2 concentration over the range from 8.3 × 10−6 M to 1.5 × 10−3 M with a correlation coefficient of 0.9962. The detection limit was found to be 2.7 × 10−6 M (S/N = 3). Furthermore, the increasing peak currents of H2O2 oxidation confirm that CuNPs had the higher catalytic ability together with polycysteine at the electrode surface. The results indicate that the modified electrode can catalyze mediated electro-oxidation of H2O2 to H2O and O2 due to the existence of Cu(II) ions according to the mechanism in Scheme 1. The higher oxidation state of Cu(III) (e.g., CuO(OH)) was believed to participate in the electro-oxidation process of alkaline solutions.To obtain optimum conditions for amperometric determination of H2O2 in flow systems, the hydrodynamic behaviour of H2O2 was investigated at the modified electrode over a potential range from −0.2 to 0.2 V. Fig. 4B shows LSVs of the modified electrode in the presence of various concentrations of H2O2. This behaviour illustrates that the oxidation of H2O2 is greatly enhanced at the modified electrode under dynamic conditions, also due to electrocatalysis. Hence, a potential of 0 V was selected as the working potential for amperometric determination of H2O2 using modified electrode under hydrodynamic conditions.
3.4 Electrocatalytic oxidation of glucose at PCFCuNP-modified electrode
The electrocatalytic activity of the modified electrode towards the oxidation of glucose in an alkaline solution was also demonstrated. Fig. 5A displays the CVs of 2.0 mM glucose in 0.10 M NaOH at the modified electrode and bare electrode, respectively. A minor signal corresponding to low oxidation with a peak potential of about +0.35 V was observed. Upon addition of 2.0 mM glucose, a single forward oxidative wave, corresponding to the irreversible glucose oxidation, was observed for the modified electrode. The oxidation process starts at approximately +0.25 V and +0.35 V vs. SCE was observed at the modified electrode, but the peak current of the modified electrode was about 2.2 times higher than that of the bare electrode. The negative shift of the peak potential and the increase in peak current for oxidation of glucose may be due to a kinetic effect by an increase in the electroactive surface area and the electron transfer ability of the modified electrode. A good linear response was obtained over a range from 6.6 × 10−6 to 1.3 × 10−3 M glucose with a correlation coefficient of 0.9983. A detection limit of 2.2 × 10−6 (S/N = 3) was observed for the determination of glucose at the modified electrode. The results indicate that the modified electrode can catalyze electro oxidation of glucose to gluconolactone due to the existence of Cu(II) ions according to the mechanism in Scheme 2.LSVs were conducted with PCFCuNP-modified electrode to investigate its electrocatalytic response under dynamic conditions. In order to obtain maximum catalytic sensitivity, the applied potential was optimized in a potential range from 0 to +0.6 V. Fig. 5B shows the LSVs of various concentrations of glucose. As can be seen, the peak current reaches a maximum at +0.35 V for glucose oxidation on modified electrode. The results are similar to that of CV curves described in Fig. 5A. In order to obtain constant, high sensitivity with a reduced overpotential for glucose oxidation, a potential of +0.35 V was applied during the amperometric determination of glucose.
3.5 Amperometric determination of H2O2 and glucose
Fig. 6 and 7 depict the amperometric current–time response of PCFCuNP-modified electrode for the determination of H2O2 and glucose. The response current was measured at a fixed potential with stirring in a 0.1 M NaOH solution. Fig. 6A shows the amperometric response obtained at an applied potential of 0 V for the successive addition of 380 mM H2O2 to a 0.1 M NaOH solution with stirring. The modified electrode shows a stepwise increase in current for every successive addition of H2O2. The modified electrode exhibited rapid response to the changes in the concentration of H2O2 (less than 4 s). The modified electrode showed a linear response to H2O2 in the concentration range from 1.6 × 10−5 to 5.0 × 10−3 M with a correlation coefficient of 0.9987. The corresponding calibration graph for the determination of H2O2 was shown in Fig. 6B. Similarly, the amperometric response for the successive additions of 200 mM glucose at a fixed potential of +0.35 V is shown in Fig. 7A. The modified electrode also shows an increase in current for every successive addition of glucose. The linear range was between 7.3 × 10−6 and 2.2 × 10−3 M, and the correlation coefficient was 0.9979. The corresponding calibration graph for the determination of glucose is shown in Fig. 7B. The detection limit (S/N = 3) for H2O2 was 5.3 × 10−6 and that for glucose was 2.4 × 10−6 M. The relative standard deviation (RSD) for eight successive determinations of 1.0 × 10−3 M H2O2 and 5.0 × 10−4 M glucose was 2.8% and 3.6%, respectively, indicating the good reproducibility of the proposed modified electrode. The novelty of this modified electrode reduced the overpotential for the detection of both H2O2 and glucose compared to the previous reports. The working potential, detection limit and linear concentration range of other related modified electrodes for H2O2 and glucose detection are reported in Table 1.| Electrode based on | Applied potential (V) | Linear range (μM) | Detection limit (μM) | Ref. |
|---|---|---|---|---|
| a MWCNT/AgNP–Au-multi-wall carbon nanotube/silver nanoparticle nanohybrids modified Au electrode.b Copper nanoparticle/poly(o-phenylenediammine) modified glassy carbon electrode.c Silver nanoparticle-modified indium tin oxide modified substrate.d Reduced graphene oxide/tyrosine-modified electrode.e Graphene oxide/Prussian Blue-modified electrode.f Polycysteine functionalized nickel nanoparticle-modified electrode.g CuO-based graphene oxide composite-modified glassy carbon electrode.h CuO nanocube-graphene nanocomposite-modified glassy carbon electrode.i Nafion/nickel oxide nanofiber-reduced graphene oxide-modified glassy carbon electrode.j CuO-modified multiwalled carbon nanotube-modified electrode. | ||||
| H2O2 | ||||
| MWCNT/AgNP-Aua | −0.20 | 50–1700 | 0.5 | 35 |
| CuO nanorod bundles | +0.25 | 100–800 | 0.2 | 36 |
| CuNP/PoPD/GCEb | −0.30 | 1–1000 | 0.1 | 37 |
| AgNP/ITOc | −0.40 | — | — | 38 |
| rGO/Tyrd | −0.55 | 100–2100 | 80 | 39 |
| GO/PBe | +0.10 | 5–1200 | 0.12 | 40 |
| PCFNiNPf | +0.50 | 3.3–1700 | 1.1 | 27 |
| PCFCuNP | 0 | 8.3–1500 | 2.7 | This work |
| Glucose | ||||
| CuO/GO/GCEg | +0.70 | 2.79–2030 | 0.69 | 41 |
| CuO-G-GCEh | +0.55 | 2–400 | 0.70 | 42 |
| CuO nanorods | +0.60 | 4–8000 | 4.0 | 43 |
| Nafion/CuO/GCE | +0.60 | 50–2550 | 1.0 | 44 |
| NA/NiONF-rGO/GCEi | +0.60 | 2–600 | 0.77 | 45 |
| CuO-MWCNTj | +0.40 | 0.4–1200 | 0.2 | 46 |
| PCFNiNP | +0.55 | 1.6–1400 | 0.5 | 27 |
| PCFCuNP | +0.35 | 6.6–1300 | 2.2 | This work |
3.6 Interference study
The selectivity and anti-interference ability of PCFCuNP-modified electrode was also evaluated by amperometric technique. Fig. 8 shows the amperometric response of the modified electrode to the injection of 82 μM H2O2 and 1 mM interfering species, including AA, DA, UA, EP, glucose and L-Cys (at a fixed potential of 0 V). It was evident that the influence of interfering species in H2O2 response was negligible because the oxidative potential of H2O2 is much less, due to the very high surface area of the CuNP, indicating high selectivity of the proposed modified electrode. Obviously, glucose electro oxidation occurs only at +0.35 V in the modified electrode. Hence, the PCFCuNP-modified electrode determined selectively towards H2O2 and glucose. The study of interfering species such as chloride ions was also tested at the PCFCuNP-modified electrode towards the determination of H2O2 and glucose. The influence of the chloride ions was evaluated in a concentration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The addition of 1.0 M NaCl with concentration of 1.7 × 10−2 M to 1.7 × 10−4 M H2O2 and glucose in the background electrolyte solution. The experimental result shows that the peak current did not alter the response of the sensor, indicating that PCFCuNP-modified electrode was not poisoned by chloride ion.
100. The addition of 1.0 M NaCl with concentration of 1.7 × 10−2 M to 1.7 × 10−4 M H2O2 and glucose in the background electrolyte solution. The experimental result shows that the peak current did not alter the response of the sensor, indicating that PCFCuNP-modified electrode was not poisoned by chloride ion.
3.7 Real sample analysis
To study the real feasibility of the PCFCuNP-modified electrode, the modified electrode was employed to determine H2O2 content in commercially available stain remover samples and glucose determination from urine samples obtained from a normal human being. The analyses were performed without special treatment for H2O2 determination and by diluting 100 times by 0.1 M NaOH for the human urine samples. The standard addition method was used for the analysis of the prepared samples. The data given in Tables 2 and 3 show satisfactory results for recovery. These results indicate the suitability of the proposed modified electrode for practical application towards online monitoring of H2O2 and glucose analysis.3.8 Stability and reproducibility
The stability and reproducibility of the PCFCuNP-modified electrode was investigated by comparing response currents of eight similarly modified electrodes. Relative standard deviation (RSD) was 3.5% at a H2O2 concentration of 0.10 mM and 3.8% at a glucose concentration of 0.10 mM. The modified electrodes were not poisoned by the oxidation product and could be used repeatedly for the determination of glucose and H2O2. The sensor was stored in an airtight container when not in use, and its sensitivity was tested every 5 days. The results demonstrated that its sensitivity was 96% of its initial sensitivity after being stored for 60 days. Good reproducibility and long-term stability of the sensor are desirable for most routine analyses of glucose and H2O2 determination. The improved stability of CuNP in electrocatalysis resulted from the protective polymer chain on the copper surface, which prevented passive layer formation and possible dissolution of the CuNP and thus efficiently reduced electrode fouling.4. Conclusion
A novel hybrid PCFCuNP-modified electrode was fabricated by a simple electrodeposition method using room temperature ionic liquid as a green electrolyte. The modified electrode resulted in excellent electrocatalytic activity for the oxidation of H2O2 and glucose selectively at very low potential. The hybrid enzyme-free electrochemical sensor showed a wide linear range and good analytical performance with short time response, good repeatability and acceptable stability. The proposed method was successfully applied to the determination of H2O2 in stain remover solutions and glucose from human urine samples with good recovery.Acknowledgements
The authors gratefully acknowledge the funding provided by the University Grants Commission (UGC), New Delhi, and the Department of Science and Technology, New Delhi, for financial assistance through the ‘PURSE’ program.Notes and references
- A. Lobnik and M. Cajlakovic, Sens. Actuators, B, 2001, 74, 194–199 CrossRef CAS.
- P. Westbroek, B. Van Hayte and E. Temmerman, Fresenius' J. Anal. Chem., 1996, 354, 405–409 CAS.
- X. Liu, Y. Xu, X. Ma and G. Li, Sens. Actuators, B, 2005, 106, 284–288 CrossRef CAS PubMed.
- E. C. Hurdis and H. Romeyn, Jr, Anal. Chem., 1954, 26, 320–325 CrossRef CAS.
- Z. Genfa, P. K. Dasgupta, W. S. Edgemond and J. N. Marx, Anal. Chim. Acta, 1991, 243, 207–216 CrossRef.
- X. S. Chai, Q. X. Hou, Q. Luo and J. Y. Zhu, Anal. Chim. Acta, 2004, 507, 281–284 CrossRef CAS PubMed.
- F. R. P. Rocha, E. R. Torralba, B. F. Reis, A. M. Rubio and M. de la Guardia, Talanta, 2005, 67, 673–677 CrossRef CAS PubMed.
- Y. Lin, X. Cui and L. Li, Electrochem. Commun., 2005, 7, 166–172 CrossRef CAS PubMed.
- K. S. Tseng, L. C. Chen and K. C. Ho, Sens. Actuators, B, 2005, 108, 738–745 CrossRef CAS PubMed.
- L. Gorton, A. Lindgren, T. Larsson, F. D. Munteanu, T. Ruzgas and I. Gazaryan, Anal. Chim. Acta, 1999, 400, 91–108 CrossRef CAS.
- J. C. Pickup, F. Hussain, N. D. Evans, O. J. Rolinski and D. J. S. Birch, Biosens. Bioelectron., 2005, 20, 2555–2565 CrossRef CAS PubMed.
- C. Chen, Q. Xie, D. Yang, H. Xiao, Y. Fu, Y. Tan and S. Yao, RSC Adv., 2013, 3, 4473–4491 RSC.
- L. C. Clark, Jr and C. Lyons, Ann. N. Y. Acad. Sci., 1962, 102, 29–45 CrossRef PubMed.
- S. J. Updike and G. P. Hicks, Nature, 1967, 214, 986–988 CrossRef CAS.
- J. Liu, A. Chou and W. Rahmat, Electroanalysis, 2005, 17, 38–46 CrossRef CAS.
- S. Park, H. Boo and T. D. Chung, Anal. Chim. Acta, 2006, 556, 46–57 CrossRef CAS PubMed.
- J. Wang, Electroanalysis, 2001, 13, 983–988 CrossRef CAS.
- Xi. Chen, G. Wu, Z. Cai, M. Oyama and X. Chen, Microchim. Acta, 2014, 181, 689–705 CrossRef CAS.
- Y. J. Yang, J. Zi and W. Li, Electrochim. Acta, 2014, 115, 126–130 CrossRef CAS PubMed.
- N. Lu, C. Shao, X. Li, T. Shen, M. Zhang, F. Miao, P. Zhang, X. Zhang, K. Wang, Y. Zhang and Y. Liu, RSC Adv., 2014, 4, 31056–31061 RSC.
- X. Xiao, M. Wang, H. Li, Y. Pan and P. Si, Talanta, 2014, 125, 366–371 CrossRef CAS PubMed.
- G. Liu, B. Zheng, Y. Jiang, Y. Cai, J. Dua, H. Yuan and D. Xiao, Talanta, 2012, 101, 24–31 CrossRef CAS PubMed.
- P. Prabhu, R. S. Babu and S. S. Narayanan, J. Solid State Electrochem., 2014, 18, 883–891 CrossRef CAS PubMed.
- D. R. S. Jeykumari and S. S. Narayanan, Biosens. Bioelectron., 2008, 23, 1404–1411 CrossRef CAS PubMed.
- K. Thenmozhi and S. S. Narayanan, Sens. Actuators, B, 2007, 125, 195–201 CrossRef CAS PubMed.
- F. Scholz and B. Lange, Trends Anal. Chem., 1992, 11, 359–367 CrossRef CAS.
- R. S. Babu, P. Prabhu and S. S. Narayanan, Talanta, 2013, 110, 135–143 CrossRef PubMed.
- S. R. K. kumar, N. Shanthi and D. D. Sarma, Phys. Rev., B, 2002, 66, 115105–115106 CrossRef.
- R. Czernuszewicz, E. Maslowsky, Jr and K. Nakamoto, Inorg. Chim. Acta, 1980, 40, 199–202 CrossRef CAS.
- C. G. Munce, G. K. Parker, S. A. Holt and G. A. Hope, Colloids Surf., A, 2007, 295, 152–158 CrossRef CAS PubMed.
- T. Laibo, J. A. Lairo and J. Lukkari, Appl. Surf. Sci., 2003, 212, 525–529 Search PubMed.
- G. Hu, Y. Ma, Y. Guo and S. Shao, Electrochim. Acta, 2008, 53, 6610–6615 CrossRef CAS PubMed.
- Y. Lu, S. Liang, M. Chen and J. Jia, J. Colloid Interface Sci., 2009, 332, 32–38 CrossRef CAS PubMed.
- D. R. Shankaran, N. Uehera and T. Kato, Anal. Bioanal. Chem., 2002, 374, 412–415 CrossRef PubMed.
- J. Song, L. Xu, C. Zhou, R. Xing, Q. Dai, D. Liu and H. Song, ACS Appl. Mater. Interfaces, 2013, 5, 12928–12934 CAS.
- L. Luo, L. Zh and Z. Wang, Bioelectrochemistry, 2012, 88, 156–163 CrossRef CAS PubMed.
- X. Wang, C. Hu, H. Liu, G. Du, X. He and Y. Xi, Sens. Actuators, B, 2010, 144, 220–225 CrossRef CAS PubMed.
- E. Reitz, W. Jia, M. Gentile, Y. Wang and Y. Lei, Electroanalysis, 2008, 20, 2482–2486 CrossRef CAS.
- Y. Zhang, Y. Wang, J. Ji and J. Wang, Sens. Actuators, B, 2012, 171–172, 580–587 CrossRef CAS PubMed.
- L.-C. Jiang and W.-D. Zhang, Biosens. Bioelectron., 2010, 25, 1402–1407 CrossRef CAS PubMed.
- W. Zhao, H. Wang, X. Qin, X. Wang, Z. Zhao, Z. Miao, L. Chen, M. Shan, Y. Fang and Q. Chen, Talanta, 2009, 80, 1029–1033 CrossRef CAS PubMed.
- C. B. McAuley, Y. Du, G. G. Wildgoose and R. G. Compton, Sens. Actuators, B, 2008, 135, 230–235 CrossRef PubMed.
- S. A. Kumar, P.-H. Lo and S.-M. Chen, J. Electrochem. Soc., 2009, 156, E118–E123 CrossRef PubMed.
- X. C. Song, X. Wang, Y. Fan, Z. Rong, M. Hao and Y. Yin, J. Nanopart. Res., 2011, 13, 5449–5455 CrossRef CAS.
- Q. Wang, M. Li, S. Szunerits and R. Boukherroub, Electroanalysis, 2014, 26, 156–163 CrossRef CAS.
- Y. Zhang, X. Sun, L. Zhu, H. Shen and N. Jia, Electrochim. Acta, 2011, 56, 1239–1245 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra04507f |
| This journal is © The Royal Society of Chemistry 2014 |