Porous Cu–BTC silica monoliths as efficient heterogeneous catalysts for the selective oxidation of alkylbenzenes†
Guo-Qiang Songa,
Ying-Xun Lua,
Qi Zhangb,
Fan Wanga,
Xiao-Kun Maa,
Xian-Feng Huang*a and
Zhi-Hui Zhang*b
aSchool of Pharmaceutical Engineering and Life Science, Changzhou University, Changzhou 213164, P. R. China. E-mail: xianfenghh@163.com
bJiangsu Key Laboratory of Advanced Catalytic Materials and Technology, Changzhou University, Changzhou 213164, P. R. China. E-mail: zhangzhjpu@gmail.com
First published on 2nd July 2014
Abstract
The microporous material Cu–BTC (1,3,5-benzenetricarboxylic acid, BTC) is a prototype metal–organic framework (MOF). In situ combination of Cu–BTC and macro-/mesoporous silica monoliths lead to an efficient catalyst for selective oxidation. The catalytic activities for the oxidation of alkylbenzenes were examined using tert-butyl hydroperoxide as the oxidant, showing that low-cost macro-/mesoporous silica supported Cu–BTC is a highly efficentive and reusable heterogeneous catalyst for the selective oxidation from alkylbenzenes to ketones with moderate to excellent yields.
Metal–organic frameworks (MOFs) are a class of crystalline materials having infinite network structures built with multitopic organic ligands and metal ions.1 The highly porous and extremely ordered materials can be used for a variety of applications ranging from gas storage over catalysis and sensing to drug release.1–8 In particular, porous MOFs are ideally suited for heterogeneous asymmetric catalyses because they impose size- and shape-selective restrictions through readily fine-tuned channels and pores and high enantioselectivity.9 Actually, the great potential of MOFs in heterogeneous catalysis demonstrated by Kim and Lin promoted the design, synthesis, and catalytic properties of many MOFs during the past decades.8,9 However, more progress has been hampered due to the lower chemical and mechanical stability of MOFs. To overcome this issue, immobilizing MOFs onto supports is very important for catalysis and also for other applications.10
As a prototype MOF, [Cu3(BTC)2] (Cu–BTC, BTC = 1,3,5-benzenetricarboxylate) (named also HKUST-1 or MOF-199) has coordinatively unsaturated Cu2+ sites, which induce attractive catalytic properties for reactions catalyzed by Lewis acids, such as Diels–Alder reactions,11 esterification,12 isomerisation of α-pinene oxide13 and Friedländer reaction.14 Recently, a well column-packing silica monolith-supported Cu–BTC catalyst was prepared and successfully applied in Friedländer reaction under continuous flow,15 which inspired us to utilize such novel catalyst in selective oxidations. Meantime, Wu et al. reported a porous metalloporphyrinic framework containing manganese ions,16 namely ZJU-18, with a 3-periodic, binodal, edge-transitive net of the same tbo topology17 and larger pore sizes comparing with those in Cu–BTC. ZJU-18 showed highly efficient for the selective catalytic oxidation of alkylbenzenes, but it is hard to be scaled up partly because of the low productivity of ligand 5,10,15,20-tetrakis(3,5-biscarboxylphenyl)porphyrin. However, selective oxidation of alkylbenzenes is a very important industrial process for the production of aromatic alcohols, aldehydes, ketones, and carboxylic acids,18 increasing the productivity and decreasing the cost of catalysts are essential for heterogeneous catalysis of such type selective oxidation.
Given the fact that stated above, in the present study we studied the benefit of combining the catalytic properties of finely divided MOF (Cu–BTC) with macro-/mesoporous silica monoliths. This Cu–BTC–silica catalyst exhibits highly effective and selective for catalytic oxidation of alkylbenzenes at 60 °C using tert-butyl hydroperoxide (TBHP) as the oxidant.
The silica monolith-supported Cu–BTC catalyst in this work was in situ synthesized through the infiltration of silica monoliths in a precursor solution of H3BTC and Cu(NO3)2 according to a modified experimental procedure15 using DMSO as the solvent instead of a DMF/water/ethanol mixture19,20 to avoid rapid nucleation and growth of MOF crystals at the outer surface of the monolith. Interestingly, concentration of the Cu–BTC precursor solution plays a key role in controlling the crystal size of Cu–BTC within the channels of porous silica monoliths, as indicated in the SEM images (Fig. 1). With molar ratio of H3BTC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(NO3)2·3H2O
Cu(NO3)2·3H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO varying from 5
DMSO varying from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 (monolith I) to 5
230 (monolith I) to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 (monolith II), crystalline Cu–BTC moiety with the size of 0.5–2.0 μm was observed inside the macroporosity of silica monolith II instead of nanosized crystalline Cu–BTC in monolith I, which was synthesized according to the literature.15 At the same time, TEM data (Fig. 2) show that ca. 10–20 nm faceted nanoparticles reside in the monolith I skeleton when using lower concentrated Cu–BTC precursor solution, which is in good agreement with the previous report.15
115 (monolith II), crystalline Cu–BTC moiety with the size of 0.5–2.0 μm was observed inside the macroporosity of silica monolith II instead of nanosized crystalline Cu–BTC in monolith I, which was synthesized according to the literature.15 At the same time, TEM data (Fig. 2) show that ca. 10–20 nm faceted nanoparticles reside in the monolith I skeleton when using lower concentrated Cu–BTC precursor solution, which is in good agreement with the previous report.15
 | ||
| Fig. 1 SEM images of Cu–BTC–SiO2 monolith I (a and b) and II (c and d) with different Cu–BTC precursor solutions. | ||
 | ||
| Fig. 2 TEM images of Cu–BTC–SiO2 monolith I (a) and II (b) with different Cu–BTC precursor solutions. | ||
TG data give the evidence of Cu–BTC homogeneous distribution within the entire monoliths prepared from two different Cu–BTC precursor solutions. Calculation of the weight losses of organic ligands indicates high concentration of precursor solution lead to high percentage of Cu–BTC within the final silica monoliths (23% for monolith II vs. 15% for monolith I). XRD patterns (Fig. 3a) confirm the successful preparation of Cu–BTC and Cu–BTC–SiO2 by the DMSO route. Nitrogen sorption study for the as-synthesized Cu–BTC–SiO2 monolith I and II after heating under vacuum at 150 °C reveals both type IV adsorption–desorption isotherms (Fig. 3b), typical of mesoporous materials. The presence of Cu–BTC inside the mesoporosity of parent silica is evidenced with a simultaneous decrease of the mesopore volume [from 1.24 for parent SiO215 to 0.919 (for I) and 0.725 cm3 g−1 (for II), respectively]. And a slight increase in the microporosity for II comparing with I is also observed (from 0.02 to 0.04 cm3 g−1). The BET surface area has a little shrink from 503 to 440 m2 g−1 as expected, since the crystal size of Cu–BTC is larger in monolith II than that in monolith I.
The oxidation of ethylbenzene was firstly examined as a typical substrate with TBHP in the presence and a catalytic amount of Cu–BTC silica monoliths in acetonitrile under ambient conditions (Table 1). GC-MS analysis after the reaction showed that the Cu–BTC–SiO2 catalyst can efficiently catalyze this chemical transformation in which ethylbenzene was quantitatively converted to acetophenone in >99% yield and >99% selectivity.
| Entry | Substrate | Product | Conv.b (%) | Select.b (%) |
|---|---|---|---|---|
| a Alkylbenzene (0.1 mmol), TBHP (0.15 mmol), catalyst (Cu–BTC silica monolith II, 0.005 mmol), acetonitrile (1.0 mL), acetic acid (0.2 mL), and water (0.2 mL) were stirred at 60 °C for 6 h.b Conversion (%) and selectivity (%) were determined by GC-MS.c Twelfth cycle, and the byproduct is 1-phenylethanol.d Monolith I as the catalyst.e No catalyst.f Silica monoliths as the catalyst.g As-synthesized Cu–BTC (0.005 mmol) as the catalyst.h As-synthesized Cu–BTC (0.02 mmol) as the catalyst. | ||||
| 1 |  |
 |
>99 | >99 |
| 2 | 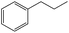 |
 |
96 | >99 |
| 3 |  |
 |
95 | >99 |
| 4 |  |
 |
29 | >99 |
| 5 |  |
 |
18 | >99 |
| 6c |  |
 |
95 | >99 |
| 7d |  |
 |
65 | >99 |
| 8e |  |
 |
Trace | — |
| 9f |  |
 |
Trace | — |
| 10g |  |
 |
39 | >99 |
| 11h |  |
 |
>99 | >99 |
A reference experiment without catalyst using the same reaction condition afforded trace production. In comparison, the as-synthesized silica monoliths also give a trace production, showing that the pure silica material is almost inactive to the oxidation reaction. After the immobilization with Cu–BTC, the two heterogeneous catalysts, i.e. Cu–BTC–SiO2 monolith I and II, demonstrate remarkably higher conversions (65% and >99%, respectively). More interestingly, the ratio of two conversions is close to the ratio of the percentages of active Cu–BTC ingredients within the SiO2 monoliths. For the Cu–BTC powder catalyst, increasing of the molar amounts from 0.005 mmol to 0.02 mmol leads to the improvement of the conversions from 39% to >99%. Bearing in the mind that the pure Cu–BTC (Entry 10, Table 1) with the corresponding nominal weight of Cu–BTC–SiO2 monolith II (Entry 1, Table 1) results in significant lower conversion, in this sense, it is reasonable to conclude that the Cu–BTC is the essentially catalytic component while the SiO2 monoliths not only play a role as a catalytic support but also optimize the catalytic performance.
Furthermore, considering the remarkable stability of the MOF onto supports, this type of Cu–BTC–SiO2 is easily recovered by filtration and subsequently used in the successive 12 cycles without significant loss in conversion (from 95% to >99%, Fig. S2†). XRD patterns of the fresh and used catalyst (indicated in Fig. 4) clearly reveal that the crystalline structure of Cu–BTC has survived after using the catalyst in the oxidation reactions. Notably, no change has occurred in the patterns, and the noise increase observed seems to be due to some pore blocking during the reaction. For the Cu–BTC powder catalyst, the recyclability experiments were carried out by increasing of the catalyst molar amounts from 0.005 mmol to 0.02 mmol. XRD pattern of Cu–BTC after the third run (Fig. S3†) indicates the maintaining of crystalline, but it was too difficult to reuse the powder catalyst without any weight loss throughout the centrifugation, washing, and activation procedures.
To verify the heterogeneity of the reaction, a filtration experiment was carried out to check for the possible occurrence of any leaching. First, after a standard reaction has taken place for 1 h with the conversion of 62% for acetophenone, the mother liquor was split into two fractions. One part of the reaction containing the catalyst was allowed to react for another 5 h until the reaction reached 100% conversion, whereas the other was filtrated to remove the solid catalyst, and the supernatant was allowed to stand for 5 h. As shown in Fig. 5, the resultant conversions after the filtration collected show no apparent increment after the removal of the catalyst. The tentative evaluation excludes the possibility of the considerable leaching of the Cu–BTC particles on silica monoliths.
 | ||
| Fig. 5 Filtration experiment for Cu–BTC–SiO2 monolith II. Conversions and selectivities are given as a function of time. | ||
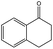
To evaluate the scope of the reaction, the oxidations of propylbenzene, 1,2,3,4-tetrahydronaphthalene, diphenyl methane, fluorene were further studied, and the substrates, propylbenzene, 1,2,3,4-tetrahydronaphthalene, were oxidized to the respective ketones in >90% yields and >99% selectivity. It is noteworthy to mention that the conversion of substrate decreases significantly when the size of the substrate increases. For example, low conversions were observed in the cases of diphenyl methane, fluorene. The much lower catalytic conversion for the larger substrates might be attributed to their difficult access to the interior pores of Cu–BTC, so the catalytic reaction mainly occurs on Cu–BTC exterior surfaces. In fact, the conversion of diphenyl methane and fluorene were very low (29% and 18%, respectively) because of the difficult access of these larger substrates to the interior pores of Cu–BTC. These results clearly indicate that catalytic properties of the catalyst for the oxidation of alkylbenzenes are substrate size-selective.
Till now, several MOFs have been reported16,18,21,22 as heterogeneous catalysts of alkylbenzenes oxidation, including two MnIII-metalloporphyrin MOFs,16,22 one CdII-9,10-anthracene dibenzoate MOF18 and one Co–BTC–formate MOF.21 Oxidation of ethylbenzene (EB) was conveniently performed as a typical experiment. All of the MOFs except the Co MOF show highly efficient and selective oxidation of alkylbenzene, with the highest conversions of >99% for ZJU-18. Notably, Cu–BTC SiO2 monolith II in this work represents the same conversion and selectivity as ZJU-18, but the effective weight is less and the reaction time is reduced than those of ZJU-18. For the two MnIII-metalloporphyrin MOFs, namely ZJU-18 and another CdII-POM-based hybrid MOF, the catalytically active sites are based on a MnIII-porphyrin16 rather than the coordination unsaturated metal sites. While for the CdII, CoII, and CuII carboxylate MOFs, all bearing unsaturated metal center after activation, the formation of t-BuOO radical as the reactive intermediate via one-electron transfer from TBHP to divalent metal ions seems to be responsible for the oxidation processes.21
In summary, an efficient catalytic material was successfully prepared by introducing Cu–BTC as microporous components within the macro-/mesoporosity of silica monoliths. The novel in situ synthesis method not only decreases the cost of preparation of MOF catalysts, but also well disperses the effective catalytic ingredients. Increasing the concentration of precursor solutions can increase the particle size. And a simple, clean and efficient catalytic oxidation procedure was also described to allow the transformation of alkylbenzenes into the corresponding ketones in excellent yield and selectivity. A plausible mechanism of these oxidation reactions is that unsaturated CuII center acts as a Lewis acid active site after coordinated H2O molecules are removed. And silica monoliths support plays a key role on the enhancement of the catalytic performance.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21201026), Youth Natural Science Foundation of Jiangsu Province (BK20130248), Natural Science Fund for Colleges and Universities in Jiangsu Province (12KJB150002), Qing Lan Project and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS PubMed.
- B. Chen, S. Xiang and G. Qian, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS PubMed.
- C. M. Doherty, D. Buso, A. J. Hill, S. Furukawa, S. Kitagawa and P. Falcaro, Acc. Chem. Res., 2014, 47, 396–405 CrossRef CAS PubMed.
- O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166–1175 CrossRef CAS PubMed.
- S. T. Meek, J. A. Greathouse and M. D. Allendorf, Adv. Mater., 2011, 23, 249–267 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- S. Qiu and G. Zhu, Coord. Chem. Rev., 2009, 253, 2891–2911 CrossRef CAS PubMed.
- L. Ma and W. Lin, in Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis, ed. M. Schroder, 2010, vol. 293, pp. 175–205 Search PubMed.
- M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed.
- D. Bradshaw, A. Garai and J. Huo, Chem. Soc. Rev., 2012, 41, 2344–2381 RSC.
- M. Heitbaum, F. Glorius and I. Escher, Angew. Chem., Int. Ed., 2006, 45, 4732–4762 CrossRef CAS PubMed.
- L. H. Wee, N. Janssens, S. R. Bajpe, C. E. A. Kirschhock and J. A. Martens, Catal. Today, 2011, 171, 275–280 CrossRef CAS PubMed.
- L. Alaerts, E. Seguin, H. Poelman, F. Thibault-Starzyk, P. A. Jacobs and D. E. De Vos, Chem.–Eur. J., 2006, 12, 7353–7363 CrossRef CAS PubMed.
- E. Perez-Mayoral and J. Cejka, ChemCatChem, 2011, 3, 157–159 CrossRef CAS.
- A. Sachse, R. Ameloot, B. Coq, F. Fajula, B. Coasne, D. De Vos and A. Galarneau, Chem. Commun., 2012, 48, 4749–4751 RSC.
- X.-L. Yang, M.-H. Xie, C. Zou, Y. He, B. Chen, M. O'Keeffe and C.-D. Wu, J. Am. Chem. Soc., 2012, 134, 10638–10645 CrossRef CAS PubMed.
- M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef PubMed.
- D. Shi, Y. Ren, H. Jiang, J. Lu and X. Cheng, Dalton Trans., 2013, 42, 484–491 RSC.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS PubMed.
- L. Hamidipour and F. Farzaneh, React. Kinet., Mech. Catal., 2013, 109, 67–75 CrossRef CAS PubMed.
- C. Zou, Z. Zhang, X. Xu, Q. Gong, J. Li and C.-D. Wu, J. Am. Chem. Soc., 2012, 134, 87–90 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, TG curves, and different runs of catalytic oxidation of ethylbenzene by Cu–BTC–SiO2 monolith II, as well as additional XRD patterns of Cu–BTC catalysts. See DOI: 10.1039/c4ra04076g |
| This journal is © The Royal Society of Chemistry 2014 |



