Development of D–π–A dyes with a pyrazine ring as an electron-withdrawing anchoring group for dye-sensitized solar cells†
Abstract
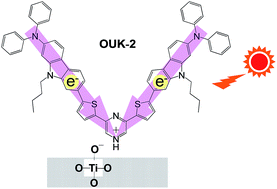
D–π–A and (D–π–)2A fluorescent dyes (OUK-1 and OUK-2) with a pyrazine ring as an electron-withdrawing anchoring group capable of forming a hydrogen bond or a pyrazinium ion at the Brønsted acid sites on TiO2 surface have been developed as a new type of D–π–A dye sensitizer for dye-sensitized solar cells.

 Please wait while we load your content...
Please wait while we load your content...