Reactions of salicylaldehydes with activated terminal alkynes in aqueous media: synthesis of 3-substituted 4-hydroxy chromenes as potential cytotoxic agents†
Shambhu Nath Singhab,
Ramu Bopannia,
Sarva Jayaprakasha,
K. Venkateshwara Reddybc,
Mohd Ashraf Ashfaqd,
K. Shiva Kumare and
Manojit Pal*d
aCustom Pharmaceuticals Services, Dr. Reddy's Laboratories Ltd, Bollaram Road, Miyapur, Hyderabad 500049, India
bDepartment of Chemistry, JNTUH College of Engineering, JNT University, Kukatpally, Hyderabad 500085, India
cCMR Engineering College Affiliated to JNTU, Medchal Road, Kandlaykoya, Hyderabad, Andhra Pradesh 501401, India
dOrganic and Medicinal Chemistry Department, Dr. Reddy's Institute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad 500046, India. E-mail: manojitpal@rediffmail.com
eDepartment of Chemistry, Osmania University, Hyderabad 500007, India
First published on 29th May 2014
Abstract
Terminal alkynes containing ester, amide or ketone moieties have been reacted with salicylaldehydes in the presence of DABCO affording a direct and single-step method for the regioselective synthesis of 3-substituted 4-hydroxy-4H-chromenes in aqueous 1,4-dioxane. A number of novel chromene derivatives were prepared in good yields using this methodology some of which showed cytotoxic properties when tested against cancer cells.
Chromenes (2H-chromenes and 4H-chromenes) constitute an important class of oxygen heterocycles and are found in many natural products.1 They also exhibit a range of pharmacological properties such as anti-HIV,2 antitumor,3 antibacterial/antimicrobial,4 fungicidal,5 and insecticidal activities.6 Additionally, this class of compounds have been well explored for the identification of potential anticancer agents.7,8 For example the chromene derivative A (Fig. 1) showed encouraging activities when tested against melanoma, prostate and glioma cancer cell lines.9 Indeed, one of them showed strong cytotoxicity in cellular assays. Prompted by these observations and due to our continuing interest in the novel and potential cytotoxic agents10 we decided to evaluate a library of small molecules based on structurally similar but simplified chromene template B (Fig. 1) for their growth inhibition potential against cancer cells.
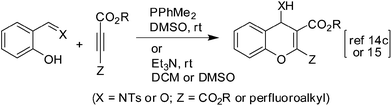
It is well known that the use of aqueous media in organic reactions not only make the process economic and environmental friendly but also enhance the reaction rate and selectivity on many occasions.11 We therefore aimed to explore the use of aqueous media in the synthesis of our target chromene derivatives B (Fig. 1). Among the several approaches reported for the construction of 4H-chromene ring two major strategies involving the use of (i) transition metal catalyzed reactions12 and (ii) organocatalysis found wide applications. While the metal catalyzed reactions have broader functional group tolerability and wider substrate scope the use of toxic metal catalysts often causes major concern. Organocatalyzed reactions on the other hand are carried out using sub-stiochiometric amounts of organic compounds and are free from the use of any inorganic materials or enzymes (e.g. biocatalysis). Organocatalysis therefore provide better alternatives to both metal and biocatalysis and emerged as a powerful tool in organic synthesis.13 Accordingly, 4-tosylamino-4H-chromene derivatives (A and B, Scheme 1) have been synthesized by Shi et al. under the conditions of organocatalysis e.g. via DABCO or phosphine-catalyzed reactions of salicyl N-tosylimines with acetylenic (e.g. but-3-yn-2-one and methyl propiolate)14a or allenic compounds (allenic esters and ketones).14b Notably, it was observed by the same group that though the amine-catalyzed reaction between ethyl-2-butynoate and salicyl N-tosylimine did not afford the corresponding chromenes (C, Scheme 1) in satisfactory yield, the reaction of diethyl acetylenedicarboxylate with salicyl N-tosylimines or salicylaldehydes proceeded smoothly (Scheme 2).14c The reaction was thought to be facilitated by the presence of electron-withdrawing ester group in place of electron-donating methyl group in ethyl 2-butynoate.14c These observations prompted Zhang and Cao et al.15 to explore the use of alkyne reactants containing highly electronegative polyfluoroalkyl groups. Thus, perfluoroalkyl containing substituted 4H-chromenes were prepared via the Et3N-catalyzed reaction of salicyl N-tosylimines or salicylaldehydes with methyl 2-perfluoroalkynoates. Interestingly, while the reaction of internal alkynes (containing electron withdrawing groups at both the sp-carbons) with salicylaldehydes leading to the 2,3-disubstituted 4-hydroxy-4H-chromenes16 has been explored the reaction of activated terminal alkynes with salicylaldehydes has not been studied earlier. Additionally, this strategy would potentially afford the 3-substituted 4-hydroxy chromene derivatives in a single step. Herein we report our preliminary results of this study (Scheme 3) and to the best of our knowledge this is the first direct synthesis of 4-hydroxy chromenes possessing substituents only at C-3 position.
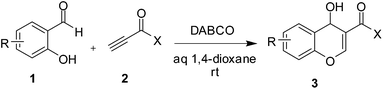 | ||
| Scheme 3 The reaction of salicylaldehydes (1) with activated terminal alkynes (2) leading to 3-substituted 4-hydroxy chromene derivatives. | ||
Initially, the reaction of commercially available 2-hydroxybenzaldehyde (1a) with ethyl propiolate (2a) was used to establish the optimum reaction conditions and the results are summarized in Table 1. The reaction was performed using the catalytic amount of DABCO in water at room temperature. The reaction was completed within 24 h affording the desired ethyl 4-hydroxy-4H-chromene-3-carboxylate (3a) in 65% yield (entry 1, Table 1). The product yield was increased (85%) considerably when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aqueous 1,4-dioxane was used (entry 2, Table 1). The use of other bases like DBU, Et3N and K2CO3 was examined but found to be less effective (entries 3–5, Table 1). Indeed, the use of K2CO3 was explored in a range of solvents (entries 6–8, Table 1) but no or poor yield of 3a was observed in these cases. Notably, a mixture of E/Z-isomers of ethyl 3-(2-formylphenoxy)acrylate was isolated as side products in >50% yield in some of these cases (entries 3–6, Table 1). These side products were thought to be formed due to an oxa-Michael addition of 1a with 2a suggesting that this might be a key step in the present synthesis of 3a. Overall, the best result was obtained using DABCO as a catalyst and 1
1 aqueous 1,4-dioxane was used (entry 2, Table 1). The use of other bases like DBU, Et3N and K2CO3 was examined but found to be less effective (entries 3–5, Table 1). Indeed, the use of K2CO3 was explored in a range of solvents (entries 6–8, Table 1) but no or poor yield of 3a was observed in these cases. Notably, a mixture of E/Z-isomers of ethyl 3-(2-formylphenoxy)acrylate was isolated as side products in >50% yield in some of these cases (entries 3–6, Table 1). These side products were thought to be formed due to an oxa-Michael addition of 1a with 2a suggesting that this might be a key step in the present synthesis of 3a. Overall, the best result was obtained using DABCO as a catalyst and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aqueous 1,4-dioxane as a solvent at room temperature. We therefore used this reaction conditions for further studies.
1 aqueous 1,4-dioxane as a solvent at room temperature. We therefore used this reaction conditions for further studies.
| Entry | Solvent | Catalyst | Time (h) | Temp (°C) | %Yieldb |
|---|---|---|---|---|---|
a Reactions were carried out using salicylaldehyde (1) (1.0 mmol), alkyne (2) (1.0 mmol), and a base (0.50 mmol) in a solvent (2.0 mL).b Isolated yield.c Ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.d A mixture of E/Z-isomers of ethyl 3-(2-formylphenoxy)acrylate was isolated as side products in >50% yield. rt = room temp. 1.d A mixture of E/Z-isomers of ethyl 3-(2-formylphenoxy)acrylate was isolated as side products in >50% yield. rt = room temp. |
|||||
| 1 | H2O | DABCO | 24 | rt | 65 |
| 2 | 1,4-Dioxane–H2Oc | DABCO | 12 | rt | 85 |
| 3 | 1,4-Dioxane–H2Oc | DBU | 12 | rt | 40d |
| 4 | 1,4-Dioxane–H2Oc | Et3N | 12 | rt | 20d |
| 5 | 1,4-Dioxane–H2Oc | K2CO3 | 24 | rt | 25d |
| 6 | THF–H2Oc | K2CO3 | 12 | 65 | 10d |
| 7 | MeCN | K2CO3 | 12 | 80 | 0 |
| 8 | THF | K2CO3 | 12 | 65 | 0 |
With the optimized reaction conditions in hand we then decided to expand the scope and generality of the present synthesis of 3-substituted 4-hydroxy chromenes. Thus a range of salicylaldehydes (1) were reacted with terminal alkynes (2) containing various carbonyl functionalities (Table 2). Substituents such as chloro and methoxy on the benzene ring of 1 were well tolerated irrespective of their position. The terminal alkynes employed may contain an ester or ketone or N-(un)substituted amide (e.g. CONH2, CONHMe, or CONHEt) moiety. The reaction proceeded well in all these cases affording the desired 3-substituted 4-hydroxy chromene 3 in good yields. The reaction was found to be highly regioselective as the formation of other regioisomer i.e. 2-substituted 4-hydroxy chromene was not detected in the reaction mixture. This was supported by the 1HNMR spectra of 3 where the C-2 proton appeared as a singlet in the region 7.6–7.9δ. In the case of other regioisomer the C-3 proton was expected to appear as a doublet (due to coupling with the C-4 proton) at a lower δ value. Nevertheless, all the compounds synthesized were characterized by spectral (NMR, IR, MS and HRMS) data.
| Entry | Salicyldehydes (R1, R2, R3 =) 1 | Alkynes (X =) 2 | Time (h) | Product 3 | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions were carried out using salicylaldehyde (1) (1.0 mmol), alkyne (2) (1.0 mmol), and DABCO (0.50 mmol) in 1,4-dioxane (1.0 mL) and water (1.0 mL) at room temperature.b Isolated yield. | |||||
| 1 | H, H, H | OEt | 12 | 3a | 85 |
| 1a | 2a | ||||
| 2 | OMe, H, H | 2a | 10 | 3b | 84 |
| 1b | |||||
| 3 | H, H, OMe | 2a | 12 | 3c | 72 |
| 1c | |||||
| 4 | H, OMe, H | 2a | 12 | 3d | 68 |
| 1d | |||||
| 5 | 1a | OMe | 10 | 3e | 82 |
| 2b | |||||
| 6 | 1b | 2b | 12 | 3f | 81 |
| 7 | 1c | 2b | 12 | 3g | 69 |
| 8 | Cl, H, H | 2a | 12 | 3h | 82 |
| 1e | |||||
| 9 | 1a | Me | 8 | 3i | 75 |
| 2c | |||||
| 10 | 1b | 2c | 8 | 3j | 78 |
| 11 | 1e | 2c | 10 | 3k | 72 |
| 12 | 1a | NH2 | 12 | 3l | 82 |
| 2d | |||||
| 13 | 1b | 2d | 12 | 3m | 80 |
| 14 | 1c | 2d | 15 | 3n | 70 |
| 15 | 1a | NHMe | 10 | 3o | 77 |
| 2e | |||||
| 16 | 1b | 2e | 12 | 3p | 78 |
Based on the earlier reports14,15 and the results of Table 1, a plausible reaction mechanism for the present synthesis of 4-hydroxy chromenes is shown in Scheme 4. The reaction involves three major steps e.g. (i) deprotonation, (ii) oxa-Michael addition, (iii) intramolecular aldol reaction and finally (iv) protonation. Thus, the salicylaldehyde (1) undergoes deprotonation in the presence of DABCO to generate the anion E-1 and the conjugate acid DABCOH+. An oxa-Michael addition of E-1 with the terminal alkyne 2 affords the intermediate E-2 which on intramolecular aldol type reaction and subsequent protonation affords the product 3 with the regeneration of the free base DABCO. Since the ionic intermediate E-3 is stabilized better in a polar protic solvent hence the intramolecular aldol reaction (a reversible step) is favoured in aqueous 1,4-dioxane. Additionally, the last proton transfer step is also expected to be faster in aqueous 1,4-dioxane. Overall, a combined effect of all these factors seemed to help the reaction proceed well in aqueous 1,4-dioxane. While the reaction also proceeded in pure water (entry 1, Table 1) the poor solubility of participating reactants in pure water perhaps affected the reaction thereby decreasing the product yield. When E-2 failed to undergo further transformation into E-3 it was simply protonated to give a mixture of E/Z-isomers of ethyl 3-(2-formylphenoxy)acrylate as observed during optimization step (entries 3–6, Table 1).
Having synthesized a range of 4-hydroxy chromenes these compounds were tested for their anti-proliferative properties against oral (CAL 27) and breast (MD-AMB-231) cancer cell lines at 10 μM using a sulphorhodamine B assay. Gemcitabine, was used as a reference compound in this assay.17 The results of some of these compounds found to be active especially against breast cancer cell lines are presented in Table 3. While the compound 3e showed growth inhibition against oral cancer cells (Table 3), most of the other compounds, for example, 3c, 3d, 3e, 3h and 3i were found to be effective against breast cancer and 3c and 3d being the best among them (Table 3). Notably, none of these compounds showed significant effects on non-cancer cells e.g. HEK 293T [Human Embryonic Kidney 293 cells] when tested at 10 μM indicating their selectivity towards cancer cells. Since breast cancer accounts for 23% of all cancers in women worldwide, hence identification of appropriate agents to fight against breast cancer is an important goal and the compounds presented here therefore may have medicinal value.
In conclusion, we have demonstrated for the first time that appropriately activated terminal alkynes can be reacted with salicylaldehyde to give 3-substituted 4-hydroxy chromenes in a regioselective manner. This reaction can be performed in the presence of DABCO in an aqueous media and a range of compounds have been prepared in good yields under the mild reaction conditions. Some of these compounds showed cytotoxic properties when tested against cancer cells especially against breast cancer. Thus the present 4-hydroxy chromene framework could be an attractive template for the identification of novel and potential agents to treat breast cancer and the synthetic methodology presented can be useful to generate a library of small molecules of medicinal value.
SNS thanks Dr Vilas Dahanukar, Dr Upadhya Timmanna and the analytical group of DRL for support.
Notes and references
- (a) E. E. Schweizer and O. Meeder-Nycz, in Chromenes, Chromanes, Chromones, ed. G. P. Ellis, Wiley-Interscience, New York, 1977 Search PubMed; (b) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS; (c) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G.-Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939 CrossRef CAS.
- (a) T. A. Engler, K. O. LaTessa, R. Iyengar, W. Chai and K. Agrios, Bioorg. Med. Chem., 1996, 4, 1755 CrossRef CAS; (b) Y. Kashiwada, K. Yamazaki, Y. Ikeshiro, T. Yamagishi, T. Fujioka, K. Mihashi, K. Mizuki, L. M. Cosentino, K. Fowke, S. L. Morris-Natschke and K. H. Lee, Tetrahedron, 2001, 57, 1559 CrossRef CAS.
- A. Elomri, S. Mitaku, S. Michel, A. L. Skaltsounis, F. Tillequin, M. Koch, A. Pierre, N. Guilbaud, S. Leonce, L. Kraus-Berthier, Y. Rolland and G. Atassi, J. Med. Chem., 1996, 39, 4762 CrossRef CAS.
- (a) M. Kidwai, S. Saxena, M. K. R. Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295 CrossRef CAS; (b) C. Tahtaoui, A. Demailly, C. Guidemann, C. Joyeux and P. Schneider, J. Org. Chem., 2010, 75, 3781 CrossRef CAS.
- J. H. G. Lago, C. S. Ramos, D. C. C. Casanova, A. A. Morandim, D. C. B. Bergamo, A. J. Cavalheiro, V. S. Bolzani, M. Furlan, E. F. Guilharaes, M. C. M. Young and M. J. Kato, J. Nat. Prod., 2004, 67, 1783 CrossRef CAS PubMed.
- C. B. Bernard, H. G. Krishnamurty, D. Chauret, T. Durst, B. J. Philogene, P. Sanchez Vindas, C. Hasbun, L. Poveda, L. San Roman and J. T. Arnason, J. Chem. Ecol., 1995, 21, 801 CrossRef CAS.
- S. X. Cai, J. Drewe and W. Kemnitzer, Anti-Cancer Agents Med. Chem., 2009, 9, 437 CrossRef CAS.
- (a) W. Kemnitzer, S. Jiang, H. Zhang, S. Kasibhatla, C. Crogan-Grundy, C. Blais, G. Attardo, R. Denis, S. Lamothe, H. Gourdeau, B. Tseng, J. Drewe and S. X. Cai, Bioorg. Med. Chem. Lett., 2008, 18, 5571 CrossRef CAS; (b) W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, C. Crogan-Grundy, D. Labreque, M. Bubenick, G. Attardo, R. Denis, S. Lamothe, H. Gourdeau, B. Tseng, S. Kasibhatla and S. X. Cai, J. Med. Chem., 2008, 51, 417 CrossRef CAS.
- S. A. Patil, J. Wang, X. S. Li, J. Chen, T. S. Jones, A. Hosni-Ahmed, R. Patil, W. L. Seibel, W. Li and D. D. Miller, Bioorg. Med. Chem. Lett., 2012, 22, 4458 CrossRef CAS.
- For our earlier effort, see: (a) N. K. Swamy, L. K. Tatini, J. M. Babu, P. Annamalai and M. Pal, Chem. Commun., 2007, 1035 RSC; (b) C. Rajitha, P. K. Dubey, V. Sunku, F. J. Piedrafita, V. R. Veeramaneni and M. Pal, Eur. J. Med. Chem., 2011, 46, 4887 CrossRef CAS; (c) N. Mulakayala, D. Rambabu, M. R. Raja, M. Chaitanya, C. S. Kumar, A. M. Kalle, R. Krishna, M. Reddy, M. V. B. Rao and M. Pal, Bioorg. Med. Chem., 2012, 20, 759 CrossRef CAS; (d) U. R. Chamakura, E. Sailaja, B. Dulla, A. M. Kalle, S. Bhavani, D. Rambabu, R. Kapavarapu, M. V. B. Rao and M. Pal, Bioorg. Med. Chem. Lett., 2014, 24, 1366 CrossRef; (e) B. Dulla, E. Sailaja, U. R. Chamakura, M. Aeluri, A. M. Kalle, S. Bhavani, D. Rambabu, M. V. B. Rao and M. Pal, Tetrahedron Lett., 2014, 55, 921 CrossRef CAS; (f) A. Nakhi, R. Adepu, D. Rambabu, R. Kishore, G. R. Vanaja, A. M. Kalle and M. Pal, Bioorg. Med. Chem. Lett., 2012, 22, 4418 CrossRef CAS.
- (a) P. Ball, H2O: A Biography of Water, Phoenix Press, London, 2000 Search PubMed; (b) C.-J. Li and T.-H. Chan, Organic reactions in aqueous media, Wiley, New York, 1997 Search PubMed.
- (a) M. Uemura, I. D. G. Watson, M. Katsukawa and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 3464 CrossRef CAS; (b) J. Fan and Z. Wang, Chem. Commun., 2008, 5381 RSC; (c) Y. Nishibayashi, Y. Inada, M. Hidai and S. Uemura, J. Am. Chem. Soc., 2002, 124, 7900 CrossRef CAS; (d) Y. Fang and C. Li, J. Org. Chem., 2006, 71, 6427 CrossRef CAS; (e) W. A. L. van Otterlo, E. L. Ngidi, S. Kuzvidza, G. L. Morgans, S. S. Moleele and C. B. de Koning, Tetrahedron, 2005, 61, 9996 CrossRef CAS; (f) S. Cacchi, D. Misiti and G. Palmieri, J. Org. Chem., 1982, 47, 2995 CrossRef CAS.
- (a) S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC; (b) M. Khoobi, L. Mamani, F. Rezazadeh, Z. Zareie, A. Foroumadi, A. Ramazani and A. Shafiee, J. Mol. Catal. A: Chem., 2012, 359, 74 CrossRef CAS.
- (a) Y. L. Shi and M. Shi, Chem.–Eur. J., 2006, 12, 3374 CrossRef CAS; (b) Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 3057 CrossRef CAS; (c) Y. W. Guo, Y.-L. Shi, H.-B. Li and M. Shi, Tetrahedron, 2006, 62, 5875 CrossRef CAS.
- L. Lu, J. Wei, J. Chen, J. Zhang, H. Deng, M. Shao, H. Zhang and W. Cao, Tetrahedron, 2009, 65, 9152 CrossRef CAS.
- (a) J. Aleman, C. Alvarado, V. Marcos, A. Nunez and J. L. G. Ruano, Synthesis, 2011, 1840 CrossRef CAS ; see also: ; (b) D. Bello, J. Ruiz-Rodríguez, F. Albericio, R. Ramón and R. Lavilla, Eur. J. Org. Chem., 2010, 2010, 5373 CrossRef.
- E. Chu and V. T. DeVita, Physicians' Cancer Chemotherapy Drug Manual, Jones & Bartlett, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, copies of the 1H and 13C NMR spectra, HRMS, and method for cytotoxicity assay. See DOI: 10.1039/c4ra03882g |
| This journal is © The Royal Society of Chemistry 2014 |