The properties and photocatalytic performance comparison of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts
Shaozheng Hua,
Ruirui Jinb,
Guang Luc,
Dan Liub and
Jianzhou Gui*b
aInstitute of Eco-environmental Sciences, Liaoning Shihua University, Fushun 113001, P.R. China
bCollege of Chemistry and Materials Science, Liaoning Shihua University, Fushun 113001, P.R. China
cSchool of Environmental and Biological Engineering, Liaoning Shihua University, Fushun 113001, P.R. China. E-mail: jzguilnpu@163.com; Tel: +86-24-56860865
First published on 20th May 2014
Abstract
Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts were prepared by a simple method using melamine and ferric nitrate as precursors. X-ray diffraction, UV-Vis spectroscopy, Fourier transform infrared spectra, scanning electron microscopy, photoluminescence, X-ray photoelectron spectroscopy and photocurrent measurements were used to characterize the prepared catalysts. The results indicated that Fe3+ was inserted at an interstitial position of g-C3N4 by coordinating to N atoms, which exist in a form completely different from those in the Fe2O3/g-C3N4 composite. The different form of Fe species caused the difference in optical properties, energy band structure and electrons–holes separation rate. The activities of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts were tested by the photocatalytic degradation of Rhodamine B (RhB) under visible light. The rate constant of Fe3+-doped g-C3N4 was 2.5 and 1.8 times higher than that of the g-C3N4 and Fe2O3/g-C3N4 composite. The possible mechanism is proposed.
Introduction
Global energy and environmental crisis is a big challenge that every country has to face. With continuous efforts among researchers, new energy sources such as solar, wind and hydrogen energy are gradually replacing traditional fossil energy sources on the stage of history. In the past decades, semiconductor photocatalysis, as a “green” technology, has been widely used for the degradation of organic compounds, treatment of toxic gases and reduction of heavy metal ions.1 However, the most commonly used metal oxide photocatalysts based on TiO2 suffer from no or limited visible light absorption due to their large band gap energies (3.0 and 3.2 eV for rutile and anatase). Therefore, most work is focused on the search of visible-light sensitive photocatalysts. One method is TiO2 modification such as by metal ion doping,2 non-metal doping,3 dye sensitization4 and semiconductor coupling.5 These approaches have resulted in improvements in visible light absorption and catalytic performance but only to a limited extent. Another method is required to develop new and efficient visible-light sensitive semiconductors.Recently, a polymeric semiconductor, graphite-like carbon nitride (g-C3N4) has attracted extensive interest. As a ‘‘sustainable’’ photocatalyst, g-C3N4 has numerous advantages such as good thermal and chemical stability, being metal-free and having a tunable electronic structure. Moreover, the moderate band gap energy (2.7 eV) allows it to directly utilize visible light. These unique properties make g-C3N4 a valuable material for various potential applications such as energy conversion,6 organic synthesis,7 pollutant treatment,8 hydrogen production9 and carbon dioxide storage.10 Nevertheless, the low efficiency caused by a high recombination rate of the photogenerated charges limits the practical application of g-C3N4.
Several routes have been developed to solve this problem such as metal and nonmetal doping,9,11–16 preparation of porous g-C3N4,17 protonating it by strong acids18 and designing composites with other semiconductors.19 Among these strategies, doping is one of the most convenient and effective methods. Zhang et al.9 synthesized I-doped g-C3N4 for hydrogen evolution. They proposed that the modification enhanced optical absorption, enlarged surface area and accelerated charge carrier transfer rate, as well as increased its hydrogen production ability. Yan et al.11 prepared B-doped g-C3N4 and suggested that the enhanced RhB degradation performance resulted from the improvement of its dye-adsorption and light-absorption ability. Zhang et al.12 synthesized phosphorus-doped g-C3N4 using an ionic liquid [Bmim]PF6 as the phosphorus source. The obtained material exhibited significantly improved electrical conductivity and photocurrent. Noble metals, such as Pt, Pd and Ag, are effective dopants that trap the photogenerated electrons, thereby improving the separation rate of photogenerated electrons and holes. Bu et al.13 prepared Ag-modified carbon nitride and found that the separation efficiency was significantly improved, thereby enhancing the photoelectric conversion performance. Chang et al.14 synthesized Pd/mpg-C3N4 and used it for photodegradation of bisphenol A. They found that most of the Pd presented in the Pd0 state acted as an electron trap, leading to the improved separation efficiency and photocatalytic performance. However, the high price of noble metals inhibits their practical application. Wang et al.15,16 found that the optical and electronic properties of g-C3N4 could also be changed by transition-metal doping such as with Fe. They suggested that Fe-doping strongly modified the electronic properties of g-C3N4 and enlarged the optical absorption range, thus leading to better photocatalytic performance.
Fe2O3, as a semiconductor with narrow band-gap energy (2.1–2.2 eV), is widely used as a visible light photocatalyst.20,21 Fe2O3 has also been used to prepare composite photocatalysts with other semiconductors, such as TiO2, Bi2O3 and SnO2, to promote charge transfer across the heterojunction, resulting in improved photocatalytic performance.22–24 However, to the best of our knowledge, few studies concerning the Fe2O3/g-C3N4 composite catalyst have been reported. The properties and photocatalytic performance of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 have also not been compared. In this work, Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts were prepared and the possible Fe doping site is discussed. The photocatalytic activity was evaluated for the degradation of RhB under visible light. The properties and photocatalytic performance of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 were compared.
Experimental
Preparation and characterization
In a typical experiment, 3 g melamine was dispersed in 15 ml deionized water under stirring. Then, 0.006–0.12 g Fe(NO3)3·9H2O was added. The obtained suspension was heated to 100 °C to remove water. The solid product was dried at 80 °C in an oven, followed by milling and annealing at 520 °C for 2 h (at a rate of 5 °C min−1). The obtained product was denoted as Fe(x%)–CN, where x is the mass percent of Fe(NO3)3·9H2O to melamine. For comparison, neat g-C3N4 was prepared following the abovementioned procedure in the absence of Fe(NO3)3·9H2O.α-Fe2O3 was prepared by a solvothermal method. Typically, 0.6 g Fe(NO3)3·9H2O and 0.5 g urea were dissolved in a mixture of glycol and distilled water. The solution was transferred into a 30 ml Teflon-lined autoclave, and then heated at 150 °C for 12 h. The solid product α-Fe2O3 was collected and washed by centrifugation. The Fe2O3/g-C3N4 composite catalyst was prepared as follows: the desired amount of α-Fe2O3 and 4.082 g melamine were dispersed in 20 ml deionized water under stirring. Then, the suspension was heated to 100 °C to remove water. The solid product was dried at 80 °C in an oven, followed by milling and annealing at 520 °C for 2 h (at a rate of 5 °C min−1). The obtained product, with the same Fe/g-C3N4 mass ratio as Fe(x%)–CN, was denoted as FeO(x%)–CN.
XRD patterns of the prepared TiO2 samples were recorded on a Rigaku D/max-2400 instrument using Cu-Kα radiation (λ = 1.54 Å). UV-Vis spectroscopy measurement was carried out on a JASCO V-550 model UV-Vis spectrophotometer using BaSO4 as the reflectance sample. FT-IR spectra were obtained on a Nicolet 20DXB FT-IR spectrometer. The morphology was observed by a scanning electron microscope (SEM, JSM 5600LV, JEOL Ltd). XPS measurements were conducted on a Thermo Escalab 250 XPS system with Al Kα radiation as the exciting source. Photoluminescence (PL) spectra were measured at room temperature with a fluorospectrophotometer (FP-6300) using an Xe lamp as excitation source. Working electrodes were prepared as follows: 0.3 g of sample was ground with 0.7 g of ethanol to prepare a slurry, and then coated on an indium–tin oxide glass by the doctor-blade method. Photocurrents were measured by electrochemical analyzer (CHI 618C Instruments) in a standard three-electrode system in which the prepared sample film was used as the working electrode, a Pt flake as the counter electrode, and Ag/AgCl as the reference electrode. A 500 W Xe-lamp was used to irradiate the working electrode from the back. A 1.0 M Na2SO4 solution was used as the electrolyte.
Photocatalytic reaction
RhB was selected as the model compound to evaluate the photocatalytic performance of the prepared g-C3N4-based catalysts in an aqueous solution under visible light irradiation. 0.05 g catalyst was dispersed in 200 ml aqueous solution of RhB (10 ppm) in an ultrasound generator for 10 min. The suspension was then transferred into a self-designed glass reactor and stirred for 30 min in darkness to achieve adsorption equilibrium. In the photoreaction under visible light irradiation, the suspension was exposed to a 250 W high-pressure sodium lamp with main emission in the range of 400–800 nm, and air was bubbled through the solution at 130 ml min−1. The UV light portion of the sodium lamp was filtered by 0.5 M NaNO2 solution. All the runs were conducted at ambient pressure and 30 °C. At given time intervals, 4 ml of the suspension was removed and immediately centrifuged to separate the liquid samples from the solid catalyst. The concentration of RhB before and after the reaction was measured by UV-Vis spectrophotometer at a wavelength of 550 nm.Results and discussion
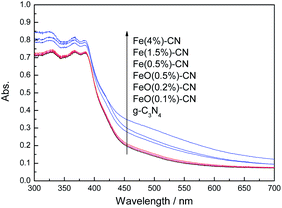
Fig. 1 shows the XRD patterns of the synthesized Fe2O3, g-C3N4, Fe(x%)–CN and FeO(x%)–CN. The patterns of g-C3N4 and Fe(x%)–CN exhibit two peaks at 13.1° and 27.3°, corresponding to (100) and (002) crystal planes of g-C3N4 (Fig. 1a), respectively.25 No peak for Fe2O3 is observed in the Fe(x%)–CN pattern, whereas an obvious shift toward a higher 2θ value (about 0.2°) is observed for all the Fe(x%)–CN catalysts. Dong et al. prepared carbon self-doping g-C3N4 and observed a similar phenomenon.26 This crystal lattice distortion may be due to the Fe3+ doping in g-C3N4. In Fig. 1b, no peak for Fe2O3 is observed in the pattern of FeO(x%)–CN, which is probably because the Fe2O3 amount is low. In addition, compared with g-C3N4, no peak shift in the pattern of FeO(x%)–CN is observed. This indicates that the existence form of Fe species differs in Fe(x%)–CN and FeO(x%)–CN.The light absorption property of the as-prepared g-C3N4, FeO(x%)–CN and Fe(x%)–CN catalysts was studied by UV-Vis spectra; the results are shown in Fig. 2. g-C3N4 shows typical semiconductor absorption, originating from charge transfer response of g-C3N4 from VB populated by N 2p orbital to CB formed by C 2p orbital.27 For FeO(x%)–CN, only a slight shift of absorption band to visible light region is observed, which is due to the low amount of Fe2O3. However, the obvious red shifts of absorption band are observed for all the Fe(x%)–CN catalysts, indicating that the band gap energies decreased. This obvious difference is due to the different forms of Fe species that exist in Fe(x%)–CN and FeO(x%)–CN. Loading of Fe2O3 did not influence the optical property of synthesized g-C3N4 because of the low Fe2O3 amount. However, Fe3+ doped into g-C3N4 lattice could affect its electronic structure, thus changing the optical property of g-C3N4. The color of the Fe(x%)–CN changed from primrose yellow to orange as the iron content increased from 0.5 to 4%. The band gap energy calculated based on the Oregan and Gratzel method indicated that the value for g-C3N4 was 2.70 eV, which is consistent with previous results.28,29 For FeO(x%)–CN, the band gap energies decreased slightly to 2.69 eV. However, for Fe(0.5%)–CN, Fe(1.5%)–CN and Fe(4%)–CN, these values were 2.53, 2.51 and 2.48 eV, respectively, indicating that Fe3+ content strongly influenced the optical property of the synthesized Fe(x%)–CN catalysts.
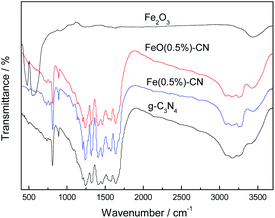
FT-IR spectra can provide plentiful structural information regarding synthesized g-C3N4-based catalysts (Fig. 3). In g-C3N4, a series of peaks ranging between 1200 and 1600 cm−1 are assigned to the typical stretching modes of CN heterocycles, whereas the sharp peak located at 810 cm−1 is assigned to the bending vibration of heptazine rings, indicating that the synthesized g-C3N4 is composed of heptazine units. The broad absorption band around 3200 cm−1 originated from the stretching vibration of the N–H bond, which was associated with uncondensed aminogroups.30 The characteristic peaks of g-C3N4 do not move in Fe(0.5%)–CN and FeO(0.5%)–CN curves, indicating that Fe modification did not change the structural features of g-C3N4. For comparison, Fe2O3 was measured (Fig. 3). The peaks at 556 and 471 cm−1 are the characteristic stretching vibrations of the Fe–O bond in hematite particles.31 These peaks are not detected in FeO(0.5%)–CN due to the low amount of Fe2O3 loading.
The morphology of the prepared g-C3N4-based catalysts was investigated by SEM. Fig. 4 shows the SEM images of the g-C3N4, FeO(0.5%)–CN and Fe(0.5%)–CN. All these catalysts exhibit a layered structure similar to that of their analogue graphite. No obvious difference was observed between g-C3N4 and FeO(0.5%)–CN, indicating that loading Fe2O3 did not influence the morphology of g-C3N4. For Fe(0.5%)–CN, the particle size decreased remarkably compared with that of g-C3N4. This indicated that Fe3+ doping could inhibit the crystal growth of graphitic carbon nitride.
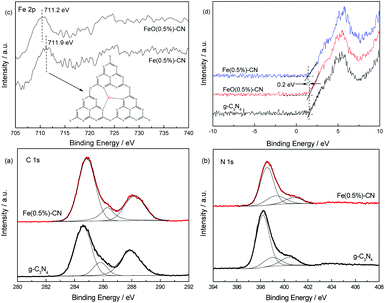
In order to confirm the structure of the prepared catalysts and to further identify the chemical state of iron, g-C3N4, FeO(0.5%)–CN and Fe(0.5%)–CN were characterized by XP spectra. In Fig. 5a and b, the spectra of g-C3N4 in both the N 1s and C 1s region can be fitted with three contributions. In the N 1s region (Fig. 5a), three contributions located at 398.2, 399.0 and 400.4 eV were assigned to the sp2 hybridized aromatic nitrogen atoms bonded to carbon atoms (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), tertiary nitrogen N–(C)3 groups linking structural motif or aminogroups carrying hydrogen ((C)2–N–H) in connection with structural defects and incomplete condensation, and nitrogen atoms bonded to three carbon atoms in the aromatic cycles.32 In Fig. 5b, three components located at 284.6, 285.8 and 287.9 eV for g-C3N4 were attributed to the C–C bond that originated from sp2 C atoms bonded to N in an aromatic ring as (N–C
C), tertiary nitrogen N–(C)3 groups linking structural motif or aminogroups carrying hydrogen ((C)2–N–H) in connection with structural defects and incomplete condensation, and nitrogen atoms bonded to three carbon atoms in the aromatic cycles.32 In Fig. 5b, three components located at 284.6, 285.8 and 287.9 eV for g-C3N4 were attributed to the C–C bond that originated from sp2 C atoms bonded to N in an aromatic ring as (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), C
N), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N or C
N or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, which could be ascribed to defect-containing sp2 hybridized carbon atoms present in graphitic domains and pure graphitic sites in a CN matrix.33,34 Fig. 5 indicates that the spectrum of Fe(0.5%)–CN in the N 1s and C 1s regions can also be fitted with three contributions. However, obvious shifts to higher binding energies were observed for Fe(0.5%)–CN in the C 1s and N 1s regions compared with that of g-C3N4. This is probably due to the change in chemical environment after Fe3+ doping. The electrons of the lone electron pair of nitrogen in g-C3N4 may be partially transferred to iron atoms, leading to the decreased electron density of nitrogen. Accordingly, the electrons on C–N bond in the g-C3N4 framework tend to be closer to the nitrogen atoms, thus the electron density of carbon atoms also decreased. These decreased electron densities cause the higher binding energies of Fe(0.5%)–CN in the C 1s and N 1s regions.
N, which could be ascribed to defect-containing sp2 hybridized carbon atoms present in graphitic domains and pure graphitic sites in a CN matrix.33,34 Fig. 5 indicates that the spectrum of Fe(0.5%)–CN in the N 1s and C 1s regions can also be fitted with three contributions. However, obvious shifts to higher binding energies were observed for Fe(0.5%)–CN in the C 1s and N 1s regions compared with that of g-C3N4. This is probably due to the change in chemical environment after Fe3+ doping. The electrons of the lone electron pair of nitrogen in g-C3N4 may be partially transferred to iron atoms, leading to the decreased electron density of nitrogen. Accordingly, the electrons on C–N bond in the g-C3N4 framework tend to be closer to the nitrogen atoms, thus the electron density of carbon atoms also decreased. These decreased electron densities cause the higher binding energies of Fe(0.5%)–CN in the C 1s and N 1s regions.
 | ||
| Fig. 5 XP spectra of synthesized g-C3N4, FeO(0.5%)–CN and Fe(0.5%)–CN in the region of C 1s (a), N 1s (b), Fe 2p (c) and VB XPS (d). | ||
In Fig. 5c, the binding energies of Fe(0.5%)–CN and FeO(0.5%)–CN in the Fe 2p region are located at 711.9 and 711.2 eV, respectively. It has been reported that the binding energies in the Fe 2p3/2 region are located at 710.8 and 711.1 eV in the formation of Fe3O4 and Fe2O3, respectively.32,35 This confirms that Fe exists as Fe2O3 in FeO(0.5%)–CN. For Fe(0.5%)–CN, this binding energy should result from Fe3+ being inserted at the interstitial position and stabilized in the electron-rich g-C3N4 structure through the Fe–N bonds (Fig. 5c inset), as reported in previous studies.16,17 The valence band XP spectra of g-C3N4, FeO(0.5%)–CN and Fe(0.5%)–CN are shown in Fig. 5d. Compared with the spectrum of g-C3N4, an obvious shift is observed in Fe(0.5%)–CN, which should be attributable to the Fe3+ doped into the g-C3N4 lattice. The binding energy difference between them is ∼0.2 eV, which is close to the calculation result from the UV-Vis spectra. No obvious binding energy difference between g-C3N4 and FeO(0.5%)–CN is revealed.
Fig. 6 shows the room temperature PL spectra of g-C3N4, FeO(0.5%)–CN and Fe(x%)–CN under the excitation wavelength of 380 nm. For g-C3N4, the broad PL band is around 465 nm, which can be attributed to the band–band PL phenomenon with the energy of light approximately equal to the band gap of g-C3N4, as calculated by UV-Vis spectra.34 Such band–band PL signal is attributed to excitonic PL, which mainly results from the n–π* electronic transitions involving lone pairs of nitrogen atoms in g-C3N4.36 FeO(x%)–CN shows a curve similar to that of g-C3N4, whereas the peak intensities decreased. In theory, the CB and VB of g-C3N4 are −1.12 V and +1.57 V, respectively.27 For Fe2O3, the CB and VB are +0.28 V and +2.48 V, respectively.37 Therefore, the interfacial charge transfer from g-C3N4 to Fe2O3 could occur, decreasing the recombination of photogenerated electron–hole pairs. The PL intensity of FeO(x%)–CN decreased at first and then increased with increasing Fe2O3 content. This indicated that over-loading Fe2O3 could increase the recombination rate of photogenerated electron–holes. For Fe(x%)–CN, the peak intensities decreased sharply. This indicates that doping is more favorable for separation of photogenerated electron–hole pairs compared with Fe2O3 loading. It is known that the reduction potential of Fe2+/Fe3+ is below the conduction band of g-C3N4.16 After Fe3+ doping, the photogenerated electrons could be trapped by the Fe3+ doping site, leading to the reduced recombination of photogenerated electron–hole pairs. With greater Fe3+ content, more photogenerated electron trapping sites exist, leading to the further decrease of PL intensity.
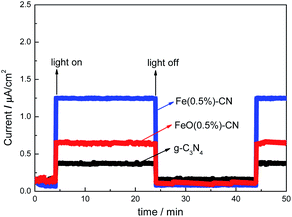
The outstanding carrier separation ability of g-C3N4-based catalysts was confirmed by the photocurrent responses measurement (I–t curve, Fig. 7). Undoubtedly, g-C3N4 exhibits a low photocurrent because of the high photogenerated electrons–holes recombination rate. The photocurrent value of FeO(0.5%)–CN is ∼1.8 times as high as that of g-C3N4, which can be attributed to the intimate interaction existing on the Fe2O3/g-C3N4 interface where photogenerated electrons and holes are efficiently separated, leading to the decreased photoinduced carrier recombination, corresponding to its enhanced photocurrent. For Fe(0.5%)–CN, the photocurrent value is ∼3 times as high as that of g-C3N4, which is much higher than that of FeO(0.5%)–CN. This indicates that Fe3+ doping could inhibit the recombination more effectively, which is consistent with PL results. In addition, the photocurrent responses did not decay with increased illumination time, indicating that the prepared catalysts could provide stable amount of electrons and holes during irradiation.
The photocatalytic performance of g-C3N4-based catalysts in the degradation of RhB under visible light irradiation is shown in Fig. 8. Control experiment results indicated that the RhB degradation performance can be ignored in the absence of either irradiation or photocatalysis, indicating that RhB was degraded via a photocatalytic process. 70% RhB was degraded over g-C3N4 in 120 min. Fe(x%)–CN showed clearly higher activities than that of g-C3N4 (Fig. 8a). This increased photocatalytic performance should be due to the synergistic effect of decreased band gap energy and reduced recombination rate of photogenerated electrons–holes pairs caused by Fe3+ doping. Fe(0.5%)–CN exhibited the highest activity of more than 99% RhB degradation in 120 min. When the Fe content exceeded 0.5%, the activity of the catalyst began to decrease; the possible reason is discussed below. In Fig. 8b, the activity of g-C3N4 increased after Fe2O3 loading. The activity increased in the order of FeO(0.1%)–CN < FeO(0.5%)–CN < FeO(0.2%)–CN, which is opposite to the PL intensity order. Considering that no obvious difference in structural and optical properties among FeO(x%)–CN catalysts was observed, such activity order should be attributable to the different photogenerated electrons–holes separation rate (Fig. 6). To investigate the catalytic stability of Fe3+-doped g-C3N4, the photocatalytic performance of Fe(0.5%)–CN was investigated in three cycles (Fig. 9). No obvious decrease in activity was observed after three cycles, indicating that prepared Fe3+-doped g-C3N4 is stable under visible-light irradiation.
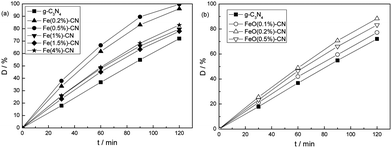 | ||
| Fig. 8 Photocatalytic performances of g-C3N4 based catalysts in the degradation of RhB under visible light irradiation. | ||
The reaction rate constant k was obtained by assuming that the reaction followed first order kinetics.38,39 In a batch reactor, the performance equation is −ln(C/C0) = kt, where C0 and C represent the concentrations of RhB dye before and after photocatalytic degradation, respectively. If a linear relationship is established when −ln(C/C0) is plotted against t (reaction time), the rate constant k can be obtained from the slope of the line. The calculated results indicated that the rate constant k was 0.0095, 0.0183, 0.0236, 0.0125, 0.0116, 0.0135, 0.0122, 0.0145 and 0.0132 min−1 for g-C3N4, Fe(0.2%)–CN, Fe(0.5%)–CN, Fe(1%)–CN, Fe(1.5%)–CN, Fe(4%)–CN, FeO(0.1%)–CN, FeO(0.2%)–CN and FeO(0.5%)–CN, respectively. Fe(0.5%)–CN exhibited the highest rate constant, which is 2.5 and 1.8 times as high as that of g-C3N4 and FeO(0.5%)–CN.
To clarify the reaction mechanism in detail, the active species generated during the reaction process were identified by a hole and free radical trapping experiment. EDTA–2Na, tert-butyl alcohol (t-BuOH) and 1,4-benzoquinone (BQ) were used as the hole (h+) scavenger, hydroxyl radical (˙OH) scavenger and superoxide radical (˙O2−) scavenger, respectively.40 Fig. 10 shows the influence of various scavengers on the photocatalytic performance of g-C3N4, Fe(0.5%)–CN and FeO–CN. For g-C3N4, the photodegradation rate of RhB decreased slightly after the addition of t-BuOH, indicating that hydroxyl radicals are not the main active species in the current photocatalytic systems. When BQ was added, the degradation of RhB decreased sharply, indicating ˙O2− is mainly responsible for the RhB degradation. In theory, the CB and VB of g-C3N4 were −1.12 V and +1.57 V, respectively. The redox potentials of ˙OH/OH− and O2/˙O2− have been determined as +1.99 V and −0.33 V, respectively.41 Therefore, the reduction potential of CB electrons in g-C3N4 was more negative than the redox potential of O2/˙O2−, which can reduce O2 to form ˙O2−, whereas the VB holes in g-C3N4 were not sufficiently positive to generate ˙OH. Therefore, the main active species in the current system should be ˙O2− and not ˙OH, which is consistent with our experimental results. In the presence of EDTA–2Na, the RhB degradation rate was obviously increased, which is completely different from previous results.40 Zhang et al.40 prepared a C3N4/Bi5Nb3O15 heterojunction catalyst for 4-CP photodegradation. The photodegradation rate decreased significantly after the addition of EDTA–2Na, indicating that the photogenerated holes were the main active species in their system. In our investigation, although the redox potential of RhB is reported to be 1.43 V,42 which is higher than the VB of g-C3N4, the direct photogenerated hole oxidation did not occur. On the contrary, the addition of EDTA–2Na to trap the h+ could promote the separation rate of e−–h+ pairs, leading to the formation of more ˙O2−. Thus, the photocatalytic performance increased. For Fe(0.5%)–CN, a similar trend has been obtained, indicating that the main oxidative species is the same as that of g-C3N4. Fe3+ doping did not change the reaction mechanism. However, when the Fe3+ content exceeded the optimal value of 0.5%, the photocatalytic performance decreased significantly, as shown in Fig. 8a, which is possibly due to competitive capture existing between the adsorbed O2 molecule and Fe3+ doping site. In this case, excess Fe3+ doping sites could trap more photogenerated electrons, leading to fewer photogenerated electrons reacting with adsorbed O2 molecules. Accordingly, the superoxide radical content reduced, thus leading to the decreased photocatalytic performance. For FeO(0.5%)–CN, the general trend is not changed but some difference is observed. When t-BuOH was added to trap ˙OH, the activity decreased by 9.4%, which was much higher than that of g-C3N4 (2.2%). The VB of Fe2O3 is +2.48 V, which is sufficiently positive to generate ˙OH. Therefore, we deduced that not only ˙O2−, but ˙OH is also one of the active species in the FeO(0.5%)–CN system. In addition, when EDTA–2Na was added to trap the holes, the activity increased (∼96%) but not as high as that of g-C3N4 (100% in 90 min). Trapping h+ could promote the separation rate of e−–h+ pairs, leading to the formation of more ˙O2−. However, the formation of ˙OH was suppressed, and therefore, the activity was lower than that of g-C3N4.
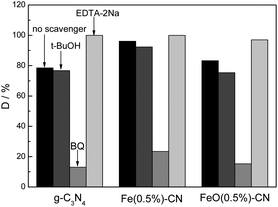 | ||
| Fig. 10 Influence of various scavengers on the visible light photocatalytic activity of g-C3N4, Fe(0.5%)–CN and FeO(0.5%)–CN. | ||
Conclusions
Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts were prepared by a simple method using melamine and ferric nitrate as precursors. Fe3+ was inserted at the interstitial position of g-C3N4 by coordinating to N atoms, which exist in a form that is completely different from a Fe2O3/g-C3N4 composite. Such a different form of Fe species caused the difference in optical properties, energy band structure and electrons–holes separation rate. The rate constant of Fe(0.5%)–CN for RhB photodegradation was 2.5 and 1.8 times higher than that of the g-C3N4 and the Fe2O3/g-C3N4 composite, respectively. When the Fe3+ content exceeded 0.5%, the activity of the catalyst began to decrease. This is due to the competitive capture existing between the adsorbed O2 molecules and the Fe3+ doping site, which suppresses the formation of ˙O2−. The superoxide radical ˙O2− was the active species in g-C3N4 and Fe3+ doped g-C3N4 systems. For Fe2O3/g-C3N4 system, both ˙O2− and ˙OH were the main active species.Acknowledgements
The authors are grateful for the financial support from Program for New Century Excellent Talents in University (NCET) (no. NCET-11-1011), the National Natural Science Foundation of China (no. 21103077), Natural Science Foundation of Liaoning Province (no. 201202123) and the Natural Science Foundation of Liaoning Shihua University (no. 2011XJJ-021).Notes and references
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- S. Klosek and D. Raftery, J. Phys. Chem. B, 2001, 105, 2815–2819 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, A. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- D. Chatterjee and A. Mahata, Appl. Catal., B, 2001, 33, 119–125 CrossRef CAS.
- S. Z. Hu, F. Y. Li, Z. P. Fan and J. Z. Gui, J. Power Sources, 2014, 250, 30–39 CrossRef CAS PubMed.
- A. Vinu, Adv. Funct. Mater., 2008, 18, 816–827 CrossRef CAS.
- J. Xu, H. T. Wu, X. Wang, B. Xue, Y. X. Li and Y. Cao, Phys. Chem. Chem. Phys., 2013, 15, 4510–4517 RSC.
- S. N. Talapaneni, S. Anandan, G. P. Mane, C. Anand, D. S. Dhawale, S. Varghese, A. Mano, T. Mori and A. Vinu, J. Mater. Chem., 2012, 22, 9831–9840 RSC.
- G. G. Zhang, M. W. Zhang, X. X. Ye, X. Q. Qiu, S. Lin and X. C. Wang, Adv. Mater., 2014, 26, 805–809 CrossRef CAS PubMed.
- Q. A. Li, J. P. Yang, D. Feng, Z. X. Wu, Q. L. Wu, S. S. Park, C. S. Ha and D. Y. Zhao, Nano Res., 2010, 3, 632–642 CrossRef CAS PubMed.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- Y. J. Zhang, T. Mori, J. H. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- Y. Y. Bu, Z. Y. Chen and W. B. Li, Appl. Catal., B, 2014, 144, 622–630 CrossRef CAS PubMed.
- C. Chang, Y. Fu, M. Hu, C. Y. Wang, G. Q. Shan and L. Y. Zhu, Appl. Catal., B, 2013, 142-143, 553–560 CrossRef CAS PubMed.
- X. F. Chen, J. S. Zhang, X. Z. Fu, M. Antonietti and X. C. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- X. C. Wang, X. F. Chen, A. Thomas, X. Z. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed.
- Y. J. Zhang, A. Thomas, M. Antonietti and X. C. Wang, J. Am. Chem. Soc., 2009, 131, 50–51 CrossRef CAS PubMed.
- C. S. Pan, J. Xu, Y. J. Wang, D. Li and Y. F. Zhu, Adv. Funct. Mater., 2012, 22, 1518–1524 CrossRef CAS.
- Q. Zhang, X. W. Lu, L. Y. Chen, Y. X. Shi, T. Xu and M. L. Liu, Mater. Lett., 2013, 106, 447–451 CrossRef CAS PubMed.
- Y. Liu, C. Y. Yu, W. Dai, X. H. Gao, H. S. Qian, Y. Hu and X. Hu, J. Alloys Compd., 2013, 551, 440–443 CrossRef CAS PubMed.
- H. Tang, D. Zhang, G. G. Tang, X. R. Ji, W. J. Li, C. S. Li and X. F. Yang, Ceram. Int., 2013, 39, 8633–8640 CrossRef CAS PubMed.
- J. Z. Li, J. B. Zhong, X. Y. He, S. T. Huang, J. Zeng, J. J. He and W. L. Shi, Appl. Surf. Sci., 2013, 284, 527–532 CrossRef CAS PubMed.
- Y. Mi, Y. H. Cao, X. L. Liu, J. B. Yi, H. R. Tan, P. Ma, H. Hao, X. Zhang and H. M. Fan, Mater. Chem. Phys., 2013, 143, 311–321 CrossRef CAS PubMed.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- G. H. Dong, K. Zhao and L. Z. Zhang, Chem. Commun., 2012, 48, 6178–6180 RSC.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- B. Oregan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- G. Q. Li, N. Yang, W. L. Wang and W. F. Zhang, J. Phys. Chem. C, 2009, 113, 14829–14833 CAS.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397–10401 CrossRef CAS PubMed.
- L. Xu, J. X. Xia, H. Xu, S. Yin, K. Wang, L. Y. Huang, L. G. Wang and H. M. Li, J. Power Sources, 2014, 245, 866–874 CrossRef CAS PubMed.
- Y. W. Zhang, J. H. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- L. Ge and C. Han, Appl. Catal., B, 2012, 117-118, 268–274 CrossRef CAS PubMed.
- W. Lei, D. Portehault, R. Dimova and M. Antoniettit, J. Am. Chem. Soc., 2011, 133, 7121–7127 CrossRef CAS PubMed.
- X. S. Zhou, B. Jin, R. Q. Chen, F. Peng and Y. P. Fang, Mater. Res. Bull., 2013, 48, 1447–1452 CrossRef CAS PubMed.
- V. N. Khabashesku, J. L. Zimmerman and J. L. Margrave, Chem. Mater., 2000, 12, 3264–3270 CrossRef CAS.
- Y. Xu and M. A. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CAS.
- C. Zhang and Y. F. Zhu, Chem. Mater., 2005, 17, 3537–3545 CrossRef CAS.
- H. B. Fu, C. S. Pan, W. Q. Yao and Y. F. Zhu, J. Phys. Chem. B, 2005, 109, 22432–22439 CrossRef CAS PubMed.
- S. Q. Zhang, Y. X. Yang, Y. N. Guo, W. Guo, M. Wang, Y. H. Guo and M. X. Huo, J. Hazard. Mater., 2013, 261, 235–245 CrossRef CAS PubMed.
- G. Liu, P. Niu, L. C. Yin and H. M. Cheng, J. Am. Chem. Soc., 2012, 134, 9070–9073 CrossRef CAS PubMed.
- T. Shen, Z. G. Zhao, Q. Yu and H. J. Xu, J. Photochem. Photobiol., A, 1989, 47, 203–212 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |