Nanoporous silica-supported organocatalyst: a heterogeneous and green hybrid catalyst for organic transformations
Sadegh Rostamnia
* and
Esmail Doustkhah
Organic and Nano Group (ONG), Department of Chemistry, Faculty of Science, University of Maragheh, P.O. Box 55181-83111, Maragheh, Iran. E-mail: rostamnia@maragheh.ac.ir; srostamnia@gmail.com; Fax: +98 421 2274893; Tel: +98 421 2278001 ext. 108
First published on 9th June 2014
Abstract
Organically modified hybrid silica mesostructures are becoming increasingly promising candidates as catalysts for various kinds of organic reactions and green chemistry. Organic moieties with an amine base and sulphonic acid have been extensively incorporated into these mesostructures. In this review, we discuss recent advances of the most common types of organically modified hybrid materials that act as organocatalysts in organic transformations and processes.
1. Introduction
Green chemistry aims to eliminate pollution by preventing it from happening in the first place and by using renewable chemical resources.1 The “greening” of global chemical processes has become a major issue in the chemical industry both in terms of selection of reactions and for the study of the effects of catalysts and of reaction mediums.1 The development of new strategies for recycling catalysts that minimize the consumption of auxiliary substances in achieving purification of the catalyst can result in significant economic and environmental benefits.1,2 Incorporating acid or base functionality into mesoporous silicas is of particular interest in organic synthesis, green chemistry and industry because of their high selectivity and high yields, and also because of the environmentally advantageous heterogeneity and reusability of these silicas.2Hybrid nanoporous materials have ever since their discovery been widely developed, particularly for adsorption, chromatography, catalysis, sensor technology, and gas storage.3,4 An advantage of these materials is that they overcome pore size limitations that occur, for example, in zeolite molecular sieves. Hybrid nanoporous materials with varied properties can be designed by incorporating specific organic moieties on or within the pore walls of their mesostructures.3,4 In addition, synthesis conditions, the template, and the type of structure directing agent (SDA) of the silica mesostructure (which has a self-assembling nature) determines the shape, thickness, and pore diameter of the mesostructure.5–8
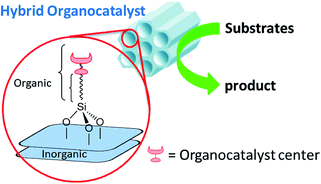
At the same time, organocatalytic reactions are becoming powerful tools for synthetic organic chemistry. The growing interest in environmentally friendly and metal-free reactions has led to great progress in organocatalysis.9–12 Moreover, much effort has gone into heterogenizing organocatalysts by immobilizing them13–15 to enable their recyclability and easy recovery. This system can deliver a green product and metal-free waste, which favors environmentally compatible processes. The purpose of this review is to discuss the scope and application of hybrid nanoporous organocatalysts as heterogeneous reusable green catalysts in transformations of organic compounds. We in particular focus on providing an overview of recent advances and developments of organocatalyst-supported hybrid materials within the silica mesostructures (Fig. 1).
2. Primary, secondary and tertiary organoamine-based mesostructures
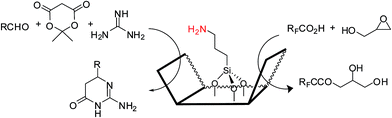
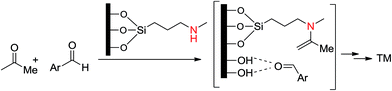
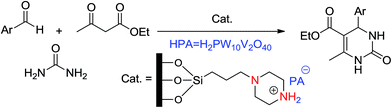
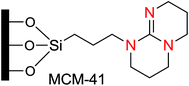
Amine-based organocatalysts are suitable and major organocatalyzing agents due to their inherent versatility in most organic reactions. They can mediate many reactions due to their nucleophilic basic nature. They can also catalyze reactions that form imines or enamines, as well as C–C and C–N bonds. Kubota and coworkers16 reviewed and reported the applications of amine-based silica mesopores in adsorption, separation, chromatography, etc. In that study, they reviewed the recent developments of various kinds of amino-grafted mesopores and discussed amino-grafted mesoporous materials as solid base catalysts and adsorbents.In 1977, Brunel et al.17 were the first to report that hybrid amino-grafted MCM-41 can act as a catalyst. In that work, they investigated a series of amine-based organocatalysts including pipyridine and (3-aminopropyl)trimethoxysilane (ATPS)-loaded MCM-41 in the synthesis of monoglycerides. Mirza-Aghayan's group18 developed the catalytic activity of ATPS-modified MCM-41 in the preparation of 2-amino-5,6-dihydropyrimidin-4(3H)-one from the cyclocondensation of Meldrum's acid, aldehydes and guanidinium carbonate (Scheme 1). Advantages of this method included good to high yields, short reaction times and ease of product isolation (Scheme 1).
Secondary amines are more efficient than primary amines in the catalysis of organic reactions.19,29 In 2002, Shimizu and coworkers used FSM-16 modified by a secondary amine in the catalysis of self-aldol condensation of aldehydes and acetone, whose activity was higher than that of a homogeneous amine catalyst.20
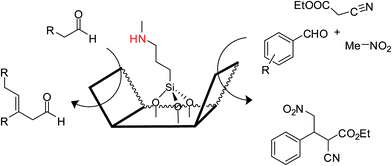
Komura et al. used aminofunctionalized MCM-41 mesoporous silica as a heterogeneous solid base catalyst. Here, a 3-methylaminopropyl moiety inside the mesoporous silica, was responsible for catalyzing a three-component one-pot reaction of a Knoevenagel condensation of an aldehyde with an active methylene compound to yield an electron-deficient alkene (Scheme 2), which underwent Michael addition of nitromethane to form a trisubstituted primary nitro compound.21
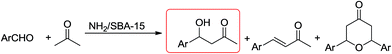
Silanol groups can act as an acidic functionality which can help form an acid–base system to improve the catalytic performance of amine-based heterogeneous mesoporous silica materials.22,23,29 Kubota et al.22 applied SBA (Santa Barbara amorphous)-15-NH2 as catalyst in the aldol reaction of 4-nitrobenzaldehyde and acetone (Scheme 3). They claimed that addition of this catalyst caused a significant increase in the rate of reaction in yield. This observation demonstrated that the physical proximity and the relative positions of two catalytic groups within the mesopore play a key role in these reactions. Silanol groups on the surface of mesoporous materials are weakly acidic, and are therefore relatively active functionally.
In 2009, Asefa et al. reported the aldol condensation reaction using MCM-41 grafted by site-isolated secondary amines onto the channel walls of mesopores.23 They found that site-isolated organoamines containing silanol groups generate a pair of molecules that exhibit acid–base cooperativity with high turnover number (TON) and selectivity (alcohol products over alkene products) during aldol condensation. In addition, when amine-based groups were grafted in the polar-protic solvent isopropanol, they showed higher catalytic activity towards aldol reactions than those grafted in the non-polar solvent toluene, due to the fact that the latter conditions cause generation of less dense amino-grafted materials loaded onto the surface of the mesopore. To elucidate the role of surface silanols as acidic sites and their ability to activate the substrates in the aldol condensation, a control experiment, with diethylamine as a homogeneous catalyst in the presence of MCM-41, silica microspheres, methyl-capped MCM-41 or methyl-capped catalyst, was carried out. Amino-grafted MCM-41 with free silanols resulted in significant enhancement of the catalytic activity compared to the corresponding reactions conducted in the absence of MCM-41, or in the presence of methyl-capped catalysts or silica spheres. By testing materials with different grafted organoamine groups as catalysts, they also found that secondary amine-functionalized samples produced the best acid and base pairs and most efficient catalytic activity in the aldol reaction. This was followed by primary amines, while the tertiary amine-functionalized samples showed negligible catalysis (Scheme 4).
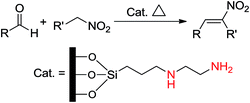
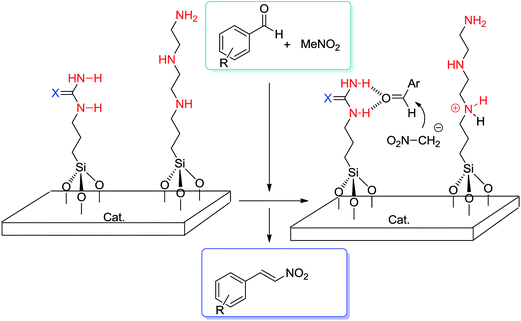
Later, Choudary et al.24 reported Knoevenagel and aldol condensations by diamino-based MCM-41 which was furnished by grafting the N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (AAPTS) on MCM-41. Then, Kantam's group reported the synthesis of nitroalkenes25 in a one-pot liquid-phase procedure using the same diamino-functionalized MCM-41 as the catalyst (Scheme 5).
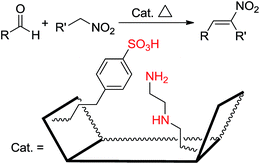
Thiel et al.26 prepared and studied a series of hybrid mesostructures organomodified by ATPS and AAPTS in a nitroaldol condensation. They found that the bifunctionalization of these amine-based mesostructures by arenesulfonic acid causes formation of a cooperative acid–base system that increases the catalytic efficiency (Scheme 6). They also compared these two amine-based functionalities in a cooperative system and found that the best yields and selectivities for nitroaldol condensation were obtained by a cooperative bifunctional system of AATPS and CSES.
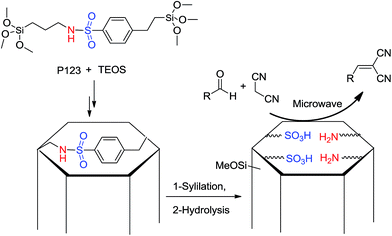
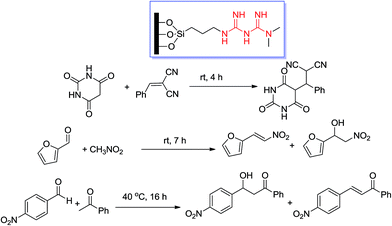
Liu et al.27 prepared the same system with a controllable acid–base bifunctionalized mesoporous catalyst with adjacent acidic and basic sites via an in situ cleavage of a sulfonamide bond in the synthesis (Scheme 7). During Knoevenagel condensation of aromatic aldehydes and ethyl cyanoacetate under microwave irradiation in solid media, the bifunctionalized mesoporous catalyst Me-A/B-SBA-15 exhibited higher catalytic activity than did the corresponding amine-functionalized catalyst and the randomly arranged acid–base catalyst, hence showing obvious acid–base cooperativity.
Based on a catalytic study of acid–base bifunctionalized systems on the mesoporous silicas by Liu,27 Tayebee et al. synthesized the heteropoly acids immobilized amine content mesoporous as HPA/amine-SBA-15 (H5PW10V2O40/Pip-SBA-15 and HPA = H5PW10V2O40). An HPA/Pip-SBA-15 heterogeneous solid catalyst was used in solvent-free conditions for the Biginelli reaction (Scheme 8). The functional groups may enhance the interactions of the heteropoly acids with the supporting material via hydrogen bonding, electrostatic interactions, and chemical bonding, resulting in greater and more stable immobilization.28
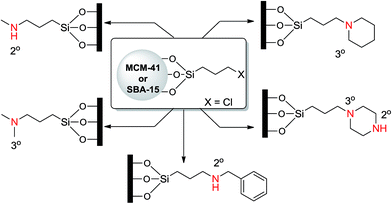
Shantz and coworkers29 prepared a series of amine-based MCM-41 materials and studied their catalytic properties in a nitroaldol reaction. They investigated the effects of organoamine type (primary, secondary, tertiary), amine density, and the presence of silanol groups on the catalytic activity and product selectivity (Scheme 9). High selectivity for the nitroalcohol product was achieved by introducing secondary and tertiary amine groups on the MCM-41 surface, while the nitroalkene was the dominant product for primary amines. The authors also claimed that the best results were obtained when the secondary amines were used. They also found that an increase in the amount of amine loading caused a decrease in the catalytic activity. One noteworthy point is that the capping of silanols with trimethylsilyl groups reduced the catalytic activity for nearly all samples, indicating the cooperative effect of surface silanols with amine groups.
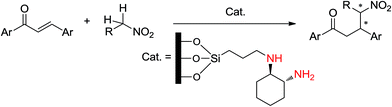
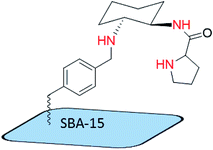
Park and coworkers30 prepared a mesopore-supported organocatalyst by a simple procedure. That is, trans-1,2-diaminocyclohexane was supported by mesoporous silica (Scheme 10) for asymmetric Michael addition of various nitroalkane derivatives. They attributed the enhancement of stereoselectivity to the plugs and short channels on the surface of mesoporous silica. They claimed that short channels and a “plug” effect resulted in higher enantioselectivity in the addition of nitroalkanes to α,β-unsaturated ketones. This reaction was performed at room temperature during 24 h with an ee value of 31–79%.
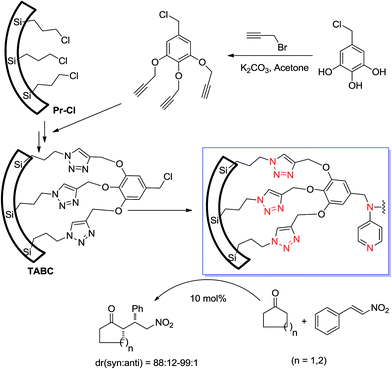
Jain and Bhaumik's research groups31 prepared an interesting heterogeneous mesoporous silica-supported organocatalyst using click chemistry. This recyclable mesoporous silica-grafted organocatalyst with a bifunctional acid–base nature was very efficient for Michael addition of ketones to β-nitrostyrenes, affording products with excellent diastereoselectivity. They found a remarkable enhancement in the reaction rates with respect to the corresponding monofunctional organocatalyst.
Preparation of the mesopore-supported organocatalyst began with functionalization of the mesopore with CPTS and substitution of azide with –Cl. Then, reaction with propargylated 5-(chloromethyl)benzene-1,2,3-triol produced TABC, which was subsequently reacted with p-aminopyridine to generate the final organocatalyst (Scheme 11).
3. Ephedrine, proline and proline-type-supported mesostructures
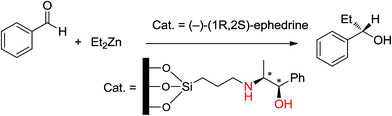
Ephedrine is a β-aminoalcohol which acts as a chiral auxiliary and organocatalyst, with its asymmetric organocatalysis having garnered interest. Abramson's group32 used (−)-ephedrine-supported Al-MTS to obtain an enantioselective catalyst to induce enantioselectivity in the alkylation of benzaldehyde by diethylzinc. (−)-Ephedrine is a chiral aminoalcohol anchored to the support through covalent bonding with 3-chloropropyltrimethoxysilane (CPTS) and substitution of the halogen by (−)-ephedrine (Scheme 12).They also studied the results obtained from changing CPTS to free silanol groups ratio in the reaction. They investigated the two kinds of and the effect of the uncovered mineral surface on activities and enantioselectivities.
A group that has a wide range of applications in organocatalysis is proline and its related compounds.33,34 Gruttadauria and coworkers recently reviewed recyclable supported proline and proline-derivatives as organocatalysts.35 In the literature, they discussed a variety of supports including the silica-supported, dendrimer-supported, polymer-supported, ionic liquid-supported, cyclodextrin-supported, and DNA-supported prolines. Conventionally, proline can be supported by either covalent bonds or electrostatic interactions.
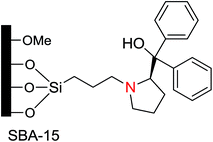
Cheng et al.36 studied the enantioselectivity of mesopore-supported chiral (S)-(−)-α,α-diphenyl-2-pyrrolidinemethanol (Fig. 2) on the same addition of diethylzinc to benzaldehyde to form (S)-1-phenyl-propanol. A proline derivative was supported on SBA-15-Pr-Cl. Enantioselective addition of diethylzinc to benzaldehyde to form (S)-1-phenyl-propanol was increased from an ee of 66 to 75% by adding a small amount of n-butyl lithium. The reaction rate and the enantioselectivity increase was attributed to the chiral proline species in the mesopores and the hydrophobicity (methylating silanol groups) around the active sites. Moreover, this chiral proline SBA-15 support was recoverable and reusable in additional cycles.
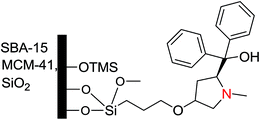
An independent research group37 studied the same reaction of diethylzinc addition to an aldehyde with a similar chiral proline derivative (Fig. 3) which was supported on SBA-15, MCM-15 and amorphous silica. Similar enantioselectivity was obtained by Cheng et al. who also increased the ee value up to 75% by adding small amount of n-butyl lithium and capping free silanols with trimethylsilyl groups.
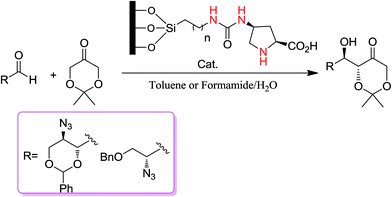
Fernández et al.38 reported the asymmetric aldol reaction of cyclic ketones and aldehydes via the heterogeneous L-proline-grafted silica MCM-41. In their report, they investigated the influence of a series of solvents on the catalysis of the asymmetric aldol reaction. Reactions proceeded more efficiently in hydrophilic polar solvents while a small amount of added water caused an increase in the rate and stereoselectivity of the reaction performed in the hydrophobic solvent toluene. The reaction under heterogeneous conditions furnished useful azasugar intermediates (Scheme 13).
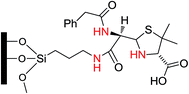
Dhar et al.39 prepared and compared MCM-41 functionalized with proline with a benzylpenicillin derivative (Fig. 4) as catalysts for a direct, asymmetric aldol reaction between acetone and activated aromatic aldehydes. In the reaction of 4-nitro and 4-fluoro benzaldehyde, the aldol products were obtained with an ee and yield of 36% and 59%, respectively. The catalysts were reusable with neither a significant drop in enantioselectivity nor loss of mesostructure. The catalysts were characterized by FT-IR, 13C CP MAS solid-state NMR, XRD and TEM techniques. Since these catalysts contain no metal, the problem of leaching encountered with many metallic catalysts does not arise. These results confirmed the proline derivative to be a more efficient catalyst with regards to yield and stereoselectivity than benzylpenicillin.
Yang et al.40 reported a similar catalyst for the asymmetric aldol reaction of cyclohexanone and p-nitrobenzaldehyde by L-prolinamide within SBA-15. This catalyst was obtained by co-condensation of TEOS, Na2SiO3, and a prolinamide group (Fig. 5). The differences between this catalyst and that reported by Dhar et al. are in the route of functionalization. The procedure includes the co-condensation of silica precursors with L-prolinamide-modified organosilane (PCA) in HOAc–NaOAc buffer solution (pH = 4.4) using block copolymer P123 as a template. A highly ordered 2D hexagonal mesostructure was obtained through a one-pot co-condensation of TEOS, Na2SiO3 and PCA as silica source. A disordered foam-like mesostructure was also obtained when TEOS and PCA were used as silicon sources. For the asymmetric aldol reaction of cyclohexanone with 4-nitrobenzaldehyde, the highly ordered mesostructures yielded higher enantioselectivity (91% ee) than the disordered foam-like mesostructure (75% ee), suggesting that the ordered pore structure imparts improved enantioselectivity. The L-prolinamide-functionalized materials were also synthesized by grafting PCA onto mesoporous silica nanoparticle (MSNs), which showed catalytic efficiencies similar to the materials synthesized by the co-condensation method.
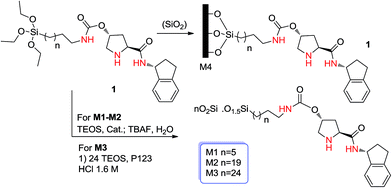
In 2012, Pleixats and coworkers41 demonstrated that amidation of the carboxylic acid moiety of proline with a suitable group can improve obtained results such as enantioselectivity. They prepared a series of catalysts (M1–M4) and tested them in the aldol reaction. They first prepared prolinamide 1 (see Scheme 14) and then, in different conditions, they obtained four kinds of catalysts. M1 was prepared by the sol–gel method and grafting on an SBA-15-type mesostructured silica. M2 and M3 were obtained by sol–gel co-condensation of prolinamide 1 with different amounts of TEOS (molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 respectively) under nucleophilic conditions using a fluoride salt (TBAF) as the catalyst. Template-assisted hydrolytic polycondensation of 1 with TEOS under acidic conditions (1.6 M HCl, P123, EtOH as co-solvent with several drops of DMF) afforded M4 ([P123] :
19 respectively) under nucleophilic conditions using a fluoride salt (TBAF) as the catalyst. Template-assisted hydrolytic polycondensation of 1 with TEOS under acidic conditions (1.6 M HCl, P123, EtOH as co-solvent with several drops of DMF) afforded M4 ([P123] :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TEOS]
[TEOS]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [3] :
[3] :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [H2O] :
[H2O] :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [HCl] :
[HCl] :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EtOH] = 0.37
[EtOH] = 0.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4390
4390![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126
126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106) (Scheme 14).
106) (Scheme 14).
The best catalytic results were obtained from a simple co-condensation without a structure-directing agent. Simple and green conditions were used for the aldol reaction, the process being performed in water at room temperature, with relatively low amounts of supported organocatalysts and in the absence of an acid co-catalyst. Good recyclabilities are observed without the need for catalyst regeneration, with enantioselectivities (ee of 82–88%) higher than that of the former free carboxylic acid prolines anchored onto MSNs (Scheme 15).
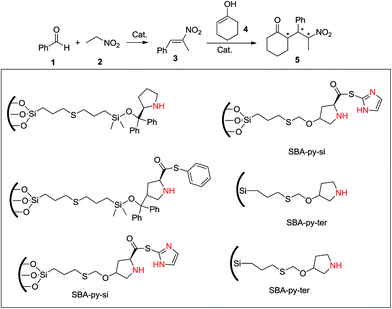
He and coworker42 designed the next acid–base hybrid catalyst system for a tandem Henry–Michael reaction, a type of aldol reaction. They used inherently achiral hydroxyls as acidic sites and immobilized chiral amines as basic sites in their bifunctional heterogeneous catalysts. A highly efficient, enantioselective and asymmetric one-pot Henry–Michael reaction was achieved using this system. Final products were obtained in yields of up to 85% and ee of 99%. (S)-2-(((Allyldimethylsilyl)oxy)diphenylmethyl)pyrrolidine, (S)-2-(((allyldimethylsilyl)oxy)methyl)pyrrolidine, (S)-(2-amino-phenyl)-4-(allyloxy)pyrrolidine-2-carbothioate and (S)-(1-methyl-1H-imidazol-2-yl)-4-(allyloxy)pyrrolidine-2-carbothioate, which all include all the pyrrolidine structure, were selected as the amine moiety. SBA-py-si-diphin catalyzed both Henry and Henry–Michael one-pot reactions. There thus appears to be a synergistic effect between the acidic hydroxyl groups and the basic grafted pyrrolidine. Without the steric hindrance of the two phenyl groups as in SBA-py-si-diph, SBA-py-si afforded 99% conversion of 1 in the heterogeneous Henry reaction and 66% yield of 5 in the heterogeneous one-pot Henry–Michael reaction. SBA-py-pri afforded a yield of 5 similar to SBA-py-si, with 74% conversion of 1 in the Henry reaction and 85% conversion of 3 in the Michael reaction. SBA-py-ter catalyzed the Michael reaction, similarly to SBA-py-pri, while catalyzing the Henry reaction more effectively than did SBA-py-pri. A yield of up to 85% for 5 was afforded on SBA-py-ter. Especially amazing and encouraging, an ee of 98% for anti-isomers and an ee of 71% for syn-isomers were afforded on SBA-py-si-diph, and >95% ee was observed for both syn- and anti-configured product on SBA-py-si, SBA-py-pri and SBA-py-ter (Scheme 16).
4. Urea, thiourea and guanidine supported onto mesostructures
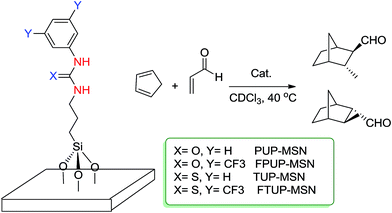
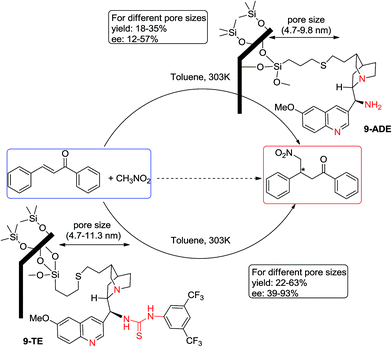
Urea and urea-type compounds form effective catalyzing agents when supported onto mesostructures. They are considered as supported organocatalysts which have been used in interesting ways to catalyze organic reactions. Lin and coworkers43 prepared a series of urea- and thiourea-functionalized MSNs and investigated their catalytic performance in the Diels–Alder reaction. In this case, the prepared catalysts exhibited a superior catalytic activity towards the Diels–Alder reaction than did homogeneous analogues. The reaction was between crotonaldehyde and cyclopentadiene (Scheme 17). They attributed the enhancement in the catalytic activity to a cooperative acid–base system. They also compared four urea- and thiourea-supported MSNs and found that among them, those containing the strong electron-withdrawing group CF3 could increase the Lewis acidity of these heterogeneous organocatalysts and thus yield the best result.He et al.44 extensively studied enantioselectivity in the Michael addition of nitromethane and chalcone. In this case they prepared several mesopores with variable pore sizes in which their outer surfaces were capped by trimethylsilyl groups and their inner surfaces were modified by propylthiols. Then they grafted two species of organocatalysts (family of epiquinine) inside the mesopores. In this research they compared heterogeneous 9-ADE and 9-TE catalysts to each other and also with their corresponding homogeneous versions. They found that the catalytic activity of 9-TE is superior to that of 9-ADE, with regards to both ee and yield. They also found a relationship between the catalytic activity of supported organocatalysts and pore size of the mesopores. The best yield and ee value for 9-ADE were obtained when pore size was set in 5.8 nm, while, for 9-TE both of ee value and yield resulted in the pore size of 6.3 nm (Scheme 18).
Urea groups adjacent to amine-based groups on the silica surface act as an acid and the amines act as a base. Therefore, they form an efficient cooperative catalytic system. Lin's group45 simultaneously incorporated AAPTS with urea on the surface of MSN and investigated it in the aldol reaction. They used a 3-ureidopropyl group as an acid and AAPTS as a base to perform aldol, Henry and cyanosilylation reactions. The turnover numbers of the bifunctionalized MSN in these reactions were higher than those of physically mixed functions with no covalent bonding to surface of the support (Fig. 6).
The successful synthesis of a series of novel biguanide-functionalized mesoporous silicas of SBA-15 catalysts was developed by Alizadeh et al.46 Various amounts of a biguanide, termed metformin, was covalently immobilized onto the silica surface. The authors investigated the structural and surface characteristics of these catalysts by various techniques such as TEM, SEM, and TGA. Then they studied their catalytic behaviors in aldol and nitroaldol coupling and Michael-addition reactions. They showed that they act as efficient and recoverable catalysts in aqueous solution with excellent reactivity combined with considerable recyclability (Scheme 19).
Ronconi and coworkers47 prepared a new amine-based MCM-41 heterogeneous mesoporous silica (Fig. 7), which is a guanidine-derived compound (1,5,7-triazabicyclo[4,4,0]dec-5-ene), and compared this MCM-41 with piperazine and ATPS-based MCM-41 in the production of biodiesel by transesterification of soybean oil in the presence of methanol. The activity of the new amine-based MCM-41 was higher than that of piperazine and ATPS-based catalysts in this reaction. The guanidine-derived catalyst generated a product yield of up to 99% at 70 °C and 3 h of reaction time, while the other amine-derived catalysts were less active for the production of biodiesel, generating lower yields under more severe reaction conditions.42
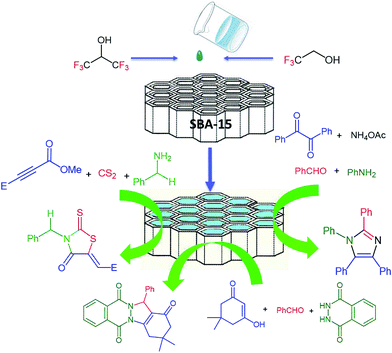
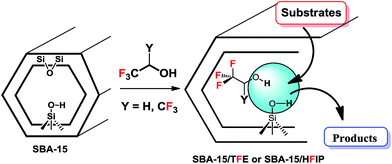
5. Fluorinated alcohol/SBA-15 adducts
One of our research interests is to develop catalytic applications of fluorinated alcohols confined in mesoporous materials.48–50 We found that the adduct of mesoporous SBA-15 and 2,2,2-trifluoroethanol (SBA-15/TFE) exhibits catalytic activity in the reaction of aldehydes, amines, and ammonia with benzil, which provides highly substituted classes of imidazoles. In this method, imidazoles, which are synthetically potential and pharmaceutically interesting compounds, were obtained via a one-pot multicomponent method. We also performed the synthesis of indazolophthalazinetrione skeletons via three-component coupling reactions in the presence of SBA-15/TFE as green conditions.50 This method has the advantage of being performed under neutral conditions without catalytic activation or a modification step (Scheme 20). This new catalytic system, which has been developed by our group, can be recovered and reused without a significant loss of the catalytic activity.48Later, we developed a similar catalytic system for the synthesis of rhodanine scaffolds, which are synthetically valuable in pharmaceuticals and industry (Scheme 21). The catalyst (SBA-15/HFIP) was obtained by confining the hexafluoroisopropanol (HFIP) inside SBA-15 at room temperature without adding additional solvent. The other advantages of this method are its low catalyst loading, simple procedure, waste-free and direct synthetic entry to excellent yields of rhodanines, high reusability of the catalyst, and short reaction time. We have not yet experimentally determined mechanisms for the syntheses of imidazoles and of rhodanine using SBA-15/TFE or SBA-15/HFIP, but a possible explanation48–50 is proposed in Scheme 21.
6. Organosulfonic acids
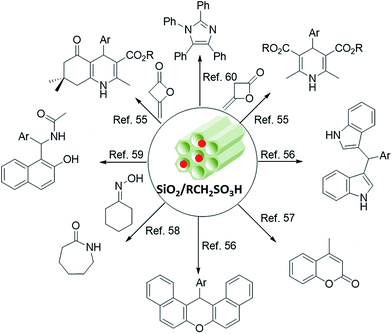
Organosulfonic acid-supported mesostructures (OSASMs) are a class of hybrid mesopores that have been extensively investigated and applied in catalysis during recent years. The pioneering reports about OSASMs were published in 1998.51–53 The earliest versions of OSASMs were generally prepared by two alternative routes: post-functionalization of 3-mercaptopropyltrimethoxysilane (MPTS) into mesoporous silica and co-condensation of MPTS with a silica source such as TEOS or TMOS during the synthesis of mesoporous silica, which is called direct synthesis of OSASMs.Since their first discovery, many types of OSASMs have been proposed and prepared, of which propylsulfonic acid-modified mesostructures have been the most common. These OASMSs have opened a great avenue of research. In 2006, Melero and coworkers54 reviewed and discussed advances, developments, and catalytic applications of OSASMs. They also overviewed new types of OSASMs synthesized from precursors other than MPTS. In addition to these primary advances in OSASMs, many catalytic applications have been developed for these hybrid catalysts, some of which are depicted in Fig. 8.55
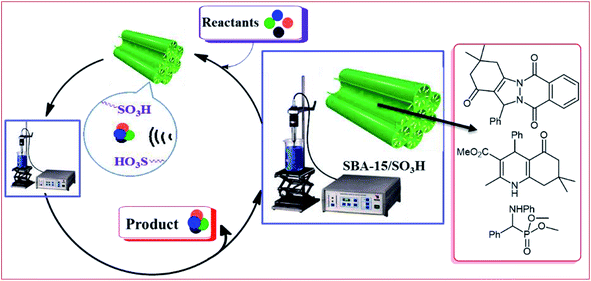
Because the silica frameworks in mesostructures have intrinsic hydrophilicity, adsorption of water may cause surface deactivation of a catalyst for mass transfer of organic molecules as substrates during reaction. Recently, we applied ultrasonication to propylsulfonic acid-grafted SBA-15 in order to increase its efficiency and to increase mass transfer within the SBA-15 pores.61 In this regard, we investigated the catalytic performance of SBA-15-Pr-SO3H in three separate biologically interesting reactions including the syntheses of 2H-indazolo[2,1-b]phthalazine-triones, α-aminophosphonates, and polyhydroquinolines (Fig. 9) under sonication. The reactions with sonication proceeded with higher selectivity and yielded products in shorter reaction times (4–25 min) than without sonication.
Another method to increase the efficiency of SBA-15-Pr-SO3H is by application of microwave irradiation. ATPS-modified MCM-41 (NH2-MCM-41) was prepared and then reacted with 1,4-butane-sultone to give MCM-41-NHSO3H.62 Afterwards, this catalytic system was used to catalyze the tert-butylation of hydroquinone while under microwave irradiation, which had a cooperative role. Moreover, amine and sulfonic acid together can also create a cooperative system. MCM-41-NHSO3H showed a high conversion of hydroquinone (88.0%) and high selectivity (93.1%) for 2-tert-butylhydroquinone (2-TBHQ) after 8 min.
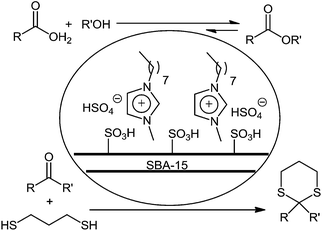
Karimi and Vafaeezadeh took another strategy to enhance the efficiency of OASMSs.63,64 They confined a hydrophobic and acidic ionic liquid (IL) within SBA-15/PrSO3H to increase its mass transfer and hydrophobicity. Moreover, this confinement caused an increase the strength of the Brønsted acid as a result of an acid site cooperativity mechanism. Their suggested catalytic system showed excellent performance in direct esterification of alcohols and carboxylic acids at ambient temperatures under solvent-free conditions.63 They also successfully incorporated this efficient catalytic system in solvent-free thioacetalization of carbonyl compounds, one of the most popular methods for protecting aldehydes and ketones, at room temperature.64 The catalyst was recoverable and reusable for at least 7 runs without a significant loss in the yield and IL (Scheme 22).
7. Conclusion
In summary, most silica mesostructure-supported organocatalysts have beneficial properties for catalysis, stereoselectivity, and green reaction delivery. Silica is the most suitable support due to its stability, functionalizing nature, and biocompatibility. Moreover, its efficiency increases when it is an ordered mesostructure. In this case, its mesochannels can act as nanoreactors, thus improving catalytic behavior, stereoselectivity, and chemoselectivity. The development of such heterogeneous organocatalysts opens the way to metal-free, stereoselective, and green chemical processes.Abbreviations
| TMOS | Tetramethyl orthosilicate |
| TEOS | Tetraethyl orthosilicate |
| MPTS | 3-Mercaptopropyltrimethoxysilane |
| AAPTS | N-(2-Aminoethyl)-3-aminopropyl trimethoxysilane |
| MPTS | 3-Mercaptopropyltrimethoxysilane |
| TM | Target molecule |
| SDA | Structure directing agent |
| CPTS | 3-Chloropropyltrimethoxysilane |
| OSASMs | Organosulphonic acid supported mesostructures |
| MSN | Mesoporous silica nanosphere |
| MSNs | Mesoporous silica nanoparticles |
| SBA | Santa Barbara amorphous |
References
- (a) N. Winterton, Chemistry for sustainable technologies: A foundation, RSC Publisher, 2010 Search PubMed; (b) M. Lancaster, Green chemistry: An introductory text, RSC Publisher, 2010 Search PubMed.
- A. Corma, D. Das, H. García and A. Leyva, J. Catal., 2005, 229, 322–331 CrossRef CAS PubMed.
- (a) C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS; (b) J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T.-W. Chu, D. H. Qlson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- (a) Q. Huo, D. I. Margolese, U. Ciesla, P. Feng, T. E. Gier, P. Sieger, R. Leon, P. M. Petroff, F. SchEth and G. D. Stucky, Nature, 1994, 368, 317–321 CrossRef CAS; (b) Q. Huo, D. I. Margolese, U. Ciesla, D. G. Demuth, P. Feng, T. E. Gier, P. Sieger, A. Firouzi, B. F. Chmelka, F. Schuth and G. D. Stucky, Chem. Mater., 1994, 6, 1176–1191 CrossRef CAS.
- T. Asefa, M. J. MacLachlan, N. Coombs and G. A. Ozin, Nature, 1999, 402, 867–871 CAS.
- B. J. Melde, B. T. Holland, C. F. Blanford and A. Stein, Chem. Mater., 1999, 11, 3302–3308 CrossRef CAS.
- S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 1999, 121, 9611–9614 CrossRef CAS.
- S. Rostamnia and E. Doustkhah, Functionalized Porous Nanoreactors in Organic Reactions, LAP-Lambert Academic publishing, 2013 Search PubMed.
- (a) P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289–296 RSC; (b) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138–5175 CrossRef CAS PubMed; (c) A. Berkessel and H. Groger, Asymmetric Organocatalysis, Wiley-VCH, Weinheim, 2005 Search PubMed.
- P. G. Bulger, Industrial Applications of Organocatalysis, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering Comprehensive Chirality, 2013, pp. 228–252 Search PubMed.
- A. B. Northrup and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 2458–2460 CrossRef CAS PubMed.
- N. A. Paras and D. W. C. MacMillan, J. Am. Chem. Soc., 2001, 123, 4370–4371 CrossRef CAS.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC.
- A. Corma, M. Iglesias, J. R. Obispo and F. Sánchez Chiral dioxo-molybdenum complexes anchored to modified usy-zeolites. Application to selective epoxidation of olefins, Chiral Reactions in Heterogeneous Catalysis, 1995, pp. 179–189 Search PubMed.
- T. Yokoi, Y. Kubota and T. Tatsumi, Appl. Catal., A, 2012, 421–422, 14–37 CrossRef CAS PubMed.
- D. Brunel, A. Cauvel, F. Fajula, F. De Renzo, A. Cauvel, G. Renard and D. Brunel, J. Org. Chem., 1997, 62, 749–751 CrossRef.
- M. Mirza-Aghayan, T. B. Lashaki, M. Rahimifard, R. Boukherroub and A. Tarlani, J. Iran. Chem. Soc., 2011, 8, 280–286 CrossRef CAS.
- K. Motokura, N. Viswanadham, G. M. Dhar and Y. Iwasawa, Catal. Today, 2009, 141, 19–24 CrossRef CAS PubMed.
- K. Shimizu, E. Hayashi, T. Inokuchi, T. Kodama, H. Hagiwara and Y. Kitayama, Tetrahedron Lett., 2002, 50, 9073–9075 CrossRef.
- K. Komura, Y. Mishima and M. Koketsu, Appl. Catal., A, 2012, 445–446, 128–132 CrossRef CAS PubMed.
- Y. Kubota, H. Yamaguchi, T. Yamada, S. Inagaki, Y. Sugi and T. Tatsumi, Top. Catal., 2010, 53, 492–499 CrossRef CAS.
- Y. Xie, K. K. Sharma, A. Anan, G. Wang, A. V. Biradar and T. Asefa, J. Catal., 2009, 265, 131–140 CrossRef CAS PubMed.
- B. M. Choudary, M. L. Kantam, P. Sreekanth, T. Bandopadhyay, F. Figueras and A. Tuel, J. Mol. Catal. A: Chem., 1999, 142, 361–365 CrossRef CAS.
- M. L. Kantam and P. Sreekanth, Catal. Lett., 1999, 57, 227–231 CrossRef CAS.
- S. Shylesh, A. Wagner, A. Seifert, S. Ernst and W. R. Thiel, Chem.–Eur. J., 2009, 15, 7052 CrossRef CAS PubMed.
- Y. Peng, J. Wang, J. Long and G. Liu, Catal. Commun., 2011, 15, 10–14 CrossRef CAS PubMed.
- R. Tayebee, M. M. Amini, M. Ghadamgahi and M. Armaghan, J. Mol. Catal. A: Chem., 2013, 366, 266–274 CrossRef CAS PubMed.
- Q. Wang and D. F. Shantz, J. Catal., 2010, 271, 170–177 CrossRef CAS PubMed.
- E.-Y. Jeong, C.-R. H. Lim and J. S.-E. Park, Chem. Commun., 2012, 48, 3079–3081 RSC.
- S. L. Jain, A. Modak and A. Bhaumik, Green Chem., 2011, 13, 586–590 RSC.
- S. Abramson, N. Bellocq and M. Laspéras, Top. Catal., 2000, 3, 339–345 CrossRef.
- (a) D. Enders and T. Gasperi, Chem. Commun., 2007, 88–90 RSC; (b) D. Enders and C. Grondal, Angew. Chem., Int. Ed., 2005, 44, 1210–1212 CrossRef CAS PubMed; (c) A. B. Northrup and D. W. C. MacMillan, Science, 2004, 1752–1755 CrossRef CAS PubMed.
- (a) C. Palomo and A. Mielgo, Angew. Chem., Int. Ed., 2006, 45, 7876–7880 CrossRef CAS PubMed; (b) C. Mitchell, A. Cobb and S. V. Ley, Synlett, 2005, 611–614 CAS; (c) S. Saito and H. Yamamoto, Acc. Chem. Res., 2004, 37, 570–579 CrossRef CAS PubMed.
- M. Gruttadauria, F. Giacalone and R. Noto, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC.
- L.-H. Hsiao, S.-Y. Chen, S.-J. Huang, S.-B. Liu, P.-H. Chen, J. C.-C. Chan and S. Cheng, Appl. Catal., A, 2009, 359, 96–107 CrossRef CAS PubMed.
- S.-W. Kim, S. J. Bae, T. Hyeon and B. M. Kim, Microporous Mesoporous Mater., 2001, 44–45, 523–529 CrossRef CAS.
- E. G. Doyagüez, F. Calderon, F. Sanchez and A. Fernández-Mayoralas, J. Org. Chem., 2007, 72, 9353–9356 CrossRef PubMed.
- D. Dhar, I. Beadham and S. Chandrasekaran, Proc. - Indian Acad. Sci., Chem. Sci., 2003, 115, 365–372 CrossRef CAS.
- J. Gao, J. Liu, D. Jiang, B. Xiao and Q. Yang, J. Mol. Catal. A: Chem., 2009, 313, 79–87 CrossRef CAS PubMed.
- A. Monge-Marcet, X. Cattoën, D. A. Alonso, C. Nájera, M. W. C. Manb and R. Pleixats, Green Chem., 2012, 14, 1601–1610 RSC.
- S. Yang and J. He, Chem. Commun., 2012, 48, 10349–10351 RSC.
- H.-T. Chen, B. G. Trewyn, J. W. Wiench, M. Pruski and V. S.-Y. Lin, Top. Catal., 2010, 53, 187–191 CrossRef CAS.
- L. Zhao, Y. Li, P. Yu, X. Han and J. He, ACS Catal., 2012, 2, 1118–1126 CrossRef CAS.
- S. Huh, H. T. Chen, J. W. Wiench, M. Pruski and S.-Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 1826–1830 CrossRef CAS PubMed.
- A. Alizadeh, M. M. Khodaei, D. Kordestani, A. H. Fallah and M. Beygzadeh, Microporous Mesoporous Mater., 2012, 159, 9–16 CrossRef CAS PubMed.
- A. L. Lima, A. Mbengue, R. A. S. S. Gil, C. M. Ronconi and C. J. A. Mota, Catal. Today, 2014, 226, 210–216 CrossRef PubMed.
- S. Rostamnia and A. Zabardasti, J. Fluorine Chem., 2012, 144, 69 CrossRef CAS PubMed.
- S. Rostamnia, E. Doustkhah and A. Nuri, J. Fluorine Chem., 2013, 153, 1–6 CrossRef CAS PubMed.
- S. Rostamnia and E. Doustkhah, Tetrahedron Lett., 2014, 55, 2508–2512 CrossRef CAS PubMed.
- V. W. M. Rhijn, D. E. De Vos, B. F. Sels and W. D. Bossaert, Chem. Commun., 1998, 317–318 RSC.
- M. H. Lim, C. F. Blanford and A. Stein, Chem. Mater., 1998, 10, 467–470 CrossRef CAS.
- W. M. Van Rhijn, D. E. De Vos, W. D. Bossaert, J. Bullen, B. Wouters, P. Grobet and P. A. Jacobs, Stud. Surf. Sci. Catal., 1998, 117, 183–190 CrossRef CAS.
- J. A. Melero, R. van Grieken and G. Morales, Chem. Rev., 2006, 106, 3790–3812 CrossRef CAS PubMed.
- S. Rostamnia and F. Pourhassan, Chin. Chem. Lett., 2013, 24, 401–403 CrossRef CAS PubMed.
- M. A. Naik, D. Sachdev and A. Dubey, Catal. Commun., 2010, 11, 1148–1153 CrossRef CAS PubMed.
- B. Karimi and D. Zareyee, Org. Lett., 2008, 10, 3989–3992 CrossRef CAS PubMed.
- X. Wang, C.-C. Chen, S.-Y. Chen, Y. Mou and S. Cheng, Appl. Catal., B, 2014, 145, 34–42 CrossRef PubMed.
- M. Hajjami, F. Ghorbani and F. Bakhti, Appl. Catal., A, 2014, 470, 303–310 CrossRef CAS PubMed.
- G. H. Mahdavinia, A. M. Amani and H. Sepehrian, Chin. J. Chem., 2012, 30, 703–708 CrossRef CAS.
- S. Rostamnia, H. Xin, X. Liu and K. Lamei, J. Mol. Catal. A: Chem., 2013, 374–375, 85–93 CrossRef CAS PubMed.
- E.-P. Ng, S. N. M. Subari, O. Marie, R. R. Mukti and J.-C. Juan, Appl. Catal., A, 2013, 450, 34–41 CrossRef CAS PubMed.
- B. Karimi and M. Vafaeezadeh, Chem. Commun., 2012, 48, 3327–3329 RSC.
- B. Karimi and M. Vafaeezadeh, RSC Adv., 2013, 3, 23207–23211 RSC.
| This journal is © The Royal Society of Chemistry 2014 |