A study of the biodegradation behaviour of poly(methacrylic acid/aniline)-grafted gum ghatti by a soil burial method
Kashma Sharmaab,
Vijay Kumar*b,
B. S. Kaithc,
Vinod Kumarb,
S. Somb,
Susheel Kalia*d and
H. C. Swart*b
aDepartment of Chemistry, Shoolini University of Biotechnology and Management Sciences, Solan, Himachal Pradesh-173212, India
bDepartment of Physics, University of the Free State, Bloemfontein ZA9300, South Africa. E-mail: vijays_phy@rediffmail.com; swarthc@ufs.ac.za; Fax: +27-514013507; Tel: +27-849774899
cDepartment of Chemistry, Dr B.R. Ambedkar National Institute of Technology, Jalandhar, Punjab-144011, India
dDepartment of Chemistry, Bahra University, Waknaghat (Shimla Hills) 173234, Dist. Solan, H.P., India. E-mail: susheel.kalia@gmail.com
First published on 20th May 2014
Abstract
Gum ghatti-based cross-linked hydrogels have been studied for their water absorption, flocculation and biodegradation properties. To date, a lot of research has been focused on gum ghatti-based cross-linked hydrogels; however, the synthesis and characterization of gum ghatti-based conductive biomaterials are relatively less explored. Moreover, the biodegradation and moisture retention studies have not been reported for conductive hydrogels. A gum ghatti-based electrically conductive hydrogel was prepared through a two-step aqueous polymerization. Biodegradation studies of the synthesized hydrogels were conducted using a soil burial test. Interpenetrating network structures showed better degradation efficiency than semi-IPN. The weight loss of semi-IPN and IPN was 66% and 86.6%, respectively, in 60 days. Different stages of degradation were studied using FTIR and SEM techniques. Furthermore, application of hydrogels to improve the water retention properties of different soils was studied for agricultural purposes, and it was found that the IPN hydrogel can improve the moisture retention capacity of soil for cultivation.
Introduction
The past two decades have witnessed the use of synthetic polymers in different fields throughout the world; however, the inherent lacuna of synthetic polymers is their non-degradability. Each year approximately 150 million tonnes of synthetic polymers are produced.1 The presence of synthetic polymers in the environment causes serious problems. Synthetic polymers are considered as major solid waste environmental pollutants. Another important issue is related to disposal of food and agricultural plastic waste. The current research interest focuses on the demand for polymeric materials that are green and eco-friendly or biodegradable in nature. Due to their excellent biocompatibility, non-toxicity and low cost, biodegradable polymers have been used in biomedical applications.2–4 The main advantages of biodegradable polymers is that they leave behind environmental friendly by-products, such as CO2, CH4 and H2O, at the end of their life cycle.5 Several biopolymers have been evaluated for their swelling behaviours, drug delivery, tissue engineering, moisture retention, biodegradability, dye removal, and metal ion adsorption.5–9 The rationales of performing these studies were to improve the properties of gum ghatti-based cross-linked hydrogels with polyaniline to increase their suitability for various applications.Graft copolymerization is an easy method to modify the structure of polysaccharides and make suitable candidates for a variety of applications.10 Gum ghatti is a naturally occurring, water soluble, and complex polysaccharide derived as an exudate from the bark of Anogeissus latifolia, a native tree of the Indian sub-continent. The morphological, structural, physico-chemical, compositional, thermal, rheological, and emulsifying properties of natural gum ghatti have been reported.11 Gum ghatti has been used in a variety of applications in the pharmaceutical, textile, paper, petroleum, and mining industries, as well as in explosives, varnishes, car polishes, ceramics and cosmetics. More recently, water absorption, flocculation and biodegradable studies of gum ghatti-based cross-linked hydrogels have been reported.5,12–14
In the pursuit of modified and improved properties of polysaccharides, recent research interest has focused towards the modification of cross-linked hydrogels with conducting polymers (CPs) via free radical polymerization, since this offers a simple technique to combine the superior properties of the CPs with the highly cross-linked hydrogels. The incorporation of CPs such as polyaniline into a flexible biopolymer matrix could result in good processability with electrical conduction, as well as chemical stability towards dopants and solubility under readily accessible conditions.15–17 CPs have been used in the microelectronics industry for application such as rechargeable lithium batteries, dye-sensitized solar cells and electrochromic displays, and more recently in biomedical applications.18 Many efforts have been made to successfully modify cross-linked hydrogels with different materials by a two-step synthetic method.15,16 Various authors have synthesized electrically conducting hydrogels based on different polysaccharides and polyaniline through graft copolymerization.17,19–21 The materials produced have found applications in the areas of biosensors and gas sensors.19,20
As a continuation of our previous work on the synthesis and characterization of conductive hydrogels based on gum ghatti using different reaction conditions4,22,23 owing to the biodegradability and biocompatibility of gum ghatti, herein we investigated the biodegradation profile of the Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline) hydrogels under soil burial conditions. Weight loss is measured gravimetrically as a function of degradation time. Changes in the chemical structure after the soil burial test are evaluated by Fourier transformed infrared analysis. Morphological changes are analysed by scanning electron microscopy. The water retention properties of the cross-linked hydrogels were also analysed in different soil samples to check their applicability in drought-prone areas. These soils were selected for their agronomic value and environmental interest. Moreover, the synthesized IPN was studied for the adsorption of a methyl orange dye in waste water, and the synthesized hydrogels were characterized using Fourier transformed infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD), and thermal gravimetric analysis (TGA) techniques. This is the first report on the synthesis of conducting hydrogels based on gum ghatti under vacuum. The reaction is carried out in vacuum in the absence of oxygen because oxygen acts as a radical inhibitor. Moreover, oxygen interferes with the progress of the reaction, which leads to the oxidation of the product and thus the yield product yield is reduced. Therefore, most of the reaction has been carried out in an inert atmosphere or in vacuum, and in the absence of oxygen the reaction proceeds uniformly without interruption and gives a better grafting yield. The synthesized conducting IPN not only has better dye adsorption behaviour but also could absorb large quantities of water and preserve the soil moisture at the same time. These were significant advantages over the semi-IPN materials.
Experimental section
Materials
Gum ghatti (Gg) [backbone] was purchased from Sigma Aldrich. Ammonium peroxydisulfate (APS) [initiator], N,N′-methylene-bis-acrylamide (MBA) [crosslinker], aniline (ANI) [monomer], methacrylic acid (MAA) [monomer], hydrochloric acid (HCl), N-methylpyrrolidone (NMP) and methyl orange (MO) [dye] were purchased from Merck, India. All the chemicals were used as received without further purification. Deionized water was used for all the reactions. The reactions were carried out in a vacuum oven at 60 °C. The reaction mixture was kept standing for different time durations and at different vacuum pressures.Synthesis of the Gg-cl-poly(MAA-IPN-aniline) conducting hydrogel
The preparation of the Gg-cl-poly(MAA-IPN-aniline) conducting hydrogel is a two-step process. The superabsorbent based on gum ghatti and methacrylic acid (MAA) was prepared using MBA as cross-linker and APS as initiator under vacuum at 60 °C for different time intervals. Details of the reaction scheme were reported in our previous work.24 Various reaction parameters such as monomer concentration, initiator concentration, cross-linker concentration, polymerization time, vacuum, amount of solvent, pH and reaction temperature were optimized to obtain the maximum percentage swelling (%S). A set of Gg-cl-poly(MAA-IPN-aniline) conducting hydrogels were also prepared following a technique that has been reported previously.22 In this study, hot air oven was replaced with a vacuum oven. For each experiment, a calculated amount of Gg-cl-poly(MAA) was added to an aqueous medium with a known amount of aniline monomer. The resulting solution was kept at room temperature for 16 h, which caused the absorption of the aniline monomer into the Gg-cl-poly(MAA) network and the formation of a swollen sample. To this mixture, a preoptimized initiator and cross-linker concentration was added with constant stirring. When a small amount of APS solution was added slowly into the solution in the polymerization step, the slightly brown color of the reaction solution changed to slightly green. The resultant mixture was placed in the vacuum oven at 60 °C to complete the polymerization process. The resulting product was washed with N-methyl-2-pyrrolidone (NMP) to remove the homopolymers. Finally, the product was dried in a vacuum oven at 50 °C and a solid Gg-cl-poly(MAA-aniline) IPN structure was obtained. Optimization was performed with respect to aniline concentration. The synthesis of the conducting IPN was carried out in a similar fashion containing 0.5 N aqueous acidic solution of HCl in the reaction flask, respectively. The conducting IPN was synthesized at various concentrations of HCl varying from 0.5–3.0 N, respectively. The synthesis outline has been depicted in Scheme 1.The percentage grafting (%G) and percentage swelling (%S) were calculated using the following equation, respectively:23
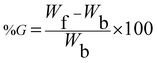 | (1) |
 | (2) |
The reactions were replicated for the reproducibility of the results and carried out in triplicate. The statistical analysis of the results was performed using the standard software package, i.e., Microsoft Excel. Arithmetic means, standard error and standard deviations were calculated. Statistical results of the optimum percentage swelling (%S) and percentage grafting (%G) are listed in Table 1.
| Sample code | Optimized reaction parameters | Mean %S | ±SD | ±SE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Backbone (g) | Initiator (mol L−1) × 10−1 | Time (min) | Amt. of solvent (ml) | pH | Monomer (mol L−1) × 10−3 | Cross-linker (mol L−1) × 10−1 | Vacuum (mm Hg) | Temp (°C) | Mean %G | ||||
| a The number of replications = 3, M = mean, ±SD = standard deviation and ±SE = standard error of the mean. | |||||||||||||
| Gg-cl-poly(MAA) | 1 | 0.131 | 150 | 12 | 7 | 0.236 | 0.324 | 450 | 60 | 60 | 1602 | 10.70 | 6.18 |
| Gg-cl-poly(MAA-aniline) | 1 | 0.131 | 150 | 12 | 7 | 0.329 | 0.324 | 450 | 60 | 29 | 1177 | 10.32 | 5.96 |
Instrumental analysis
X-ray studies were carried out on a Philips Model: PW 1830 X-ray diffractometer using Nickel-filtered Cu-Kα radiation and scanned from 15° to 50° at a scan speed of 2° min−1. The characteristic functional groups of gum ghatti and its amalgamated structures were analysed by a Nicolet 6700 FTIR spectrophotometer. TGA curves were obtained by a TGA/SDTA 851e (METTLER TOLEDO) in an argon atmosphere at a heating rate of 10 °C min−1. Scanning electron microscopy (SEM) images were obtained using a JEOL JSM-6490LV microscope at 25 kV after being covered with a thin layer (∼20 nm) of sputtered gold. The current–voltage measurements were obtained using compressed circular pellets of these materials (mass ∼0.2 g, diameter = 8 mm, thickness = 1 ± 0.07 mm, pressure = 8 ton cm−2) by a two probe method using a Keithley source meter (Model 2400).Soil burial degradation test
The biodegradability test was carried out in flower pots containing agricultural soil under moisture-controlled conditions. The hydrogel samples were buried in soil contained in the pots at a 2 cm depth and the pots were maintained at room temperature. The soil was kept moist with water after every alternate day to overcome water loss by evaporation. The samples were removed for testing their biodegradation after regular intervals of 6 days. After removal, they were cleaned properly, washed with distilled water, and dried in a vacuum oven until a constant weight. The degree of degradation at each stage was determined by evaluating the change in physical appearance, morphology, percentage weight loss, and chemical structure by FTIR spectroscopy. The weight loss of the cross-linked hydrogels was calculated by weighing the sample on an analytical balance (accuracy ± 0.00001 g) before and after degradation testing at every regular interval (6 days). The weight loss of the hydrogels with time was used to indicate the degradation rate in the soil burial test. The percentage weight loss after particular time interval was determined as follows:25
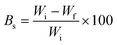 | (3) |
Water-retention studies
The water-retention studies were performed in different dry soil samples such as clay soil, sand and a mixture of both (i.e. clay soil + sand). Each sample (1.0 g) was mixed with 40 g of different soil samples, placed in a plastic beaker and then 20 ml of tap water was slowly added into the beakers and weighed (W1) using an analytical balance (accuracy ± 0.00001 g). A controlled experiment, i.e. without cross-linked hydrogels, was also carried out with different soil samples. The beakers were maintained at room temperature and were weighed everyday (Wi) until no detectable weight loss was observed. The water evaporation ratio (W%) of different soil samples was calculated using the following equation:7
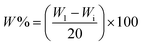 | (4) |
Dye adsorption test
Adsorption tests of methylene orange (MO) on the IPN hydrogel were carried out using UV-vis spectroscopy. For each experiment, 200 mg of the adsorbent was taken, and each adsorbent was immersed in a 20 ml aqueous solution of MO dye containing 20 ppm solution at a room temperature for 18 h. At the end of 3 h, the adsorbent was removed by simple filtration. The filtrates were analysed for the residual dye using UV-vis spectroscopy. Spectrophotometric measurements were carried out using a U-3300 UV-visible spectrophotometer at ambient temperature. The absorbance spectra were recorded in the range of 300–600 nm. Distilled water was used as the reference. The following equation was used to calculate the percentage of adsorption:4
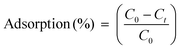 | (5) |
Results and discussion
Mechanism
In the present investigation, a graft copolymer of gum ghatti with methacrylic acid (MAA) was synthesized by a free-radical copolymerization in an aqueous media with optimized process parameters using N,N′-methylene-bis-acrylamide (MBA) as a cross-linker and ammonium persulfate (APS) as an initiator system under vacuum. –OH and –CH groups present on gum ghatti are the active sites for the synthesis of cross-linked graft copolymers. The reactive group where the grafting is initiated on Gg backbone is CH2OH. The mechanism of the formation of the copolymers has been reported elsewhere.24 Various authors have reported the free radical copolymerization of vinyl monomers with carbohydrate polymers using peroxydisulfate. A chain mechanism is involved in this reaction due to the formation of sulphate ion radicals, which are well known ion chain carriers for the graft copolymerization.4,20,26 Sulfate ions are primary radicals, which are generated from the APS by the reduction of one electron.22 The grafting of poly(MAA) onto Gg was then initiated by the free radical reacting with the monomer. In the presence of a cross-linker, the Gg free radicals are chemically combined to the monomer unit, thereby resulting in a covalent bond between the poly(MAA) and Gg to create the chain reaction for propagation.10 Finally, termination was carried out through a combination of two radicals. Note that the grafting efficiency was found to be 60%. Moreover, the Gg-cl-poly(MAA) network will serve effectively as a medium for the graft copolymerization of the aniline monomer in the presence of the MBA and APS as a cross-linker initiator system. At the same time, peroxysulfate stimulates the oxidative polymerization reaction of aniline via a medium of cationic radicals and form PANI and PANI radicals as reported earlier.22 When a fully swollen semi-IPN is placed into the aqueous aniline solution for 16 h, a form of loosely bound network is formed between the semi-IPN and aniline monomers. Finally, the semi-IPN radical and PANI cation radicals are combined to form the Gg-cl-poly(MAA-IPN-aniline) graft copolymer. Note that the mean grafting efficiency in the case of Gg-cl-poly(MAA-IPN-aniline) was found to be 29%.Optimization of various reaction parameters for the synthesis of Gg-cl-poly(MAA)
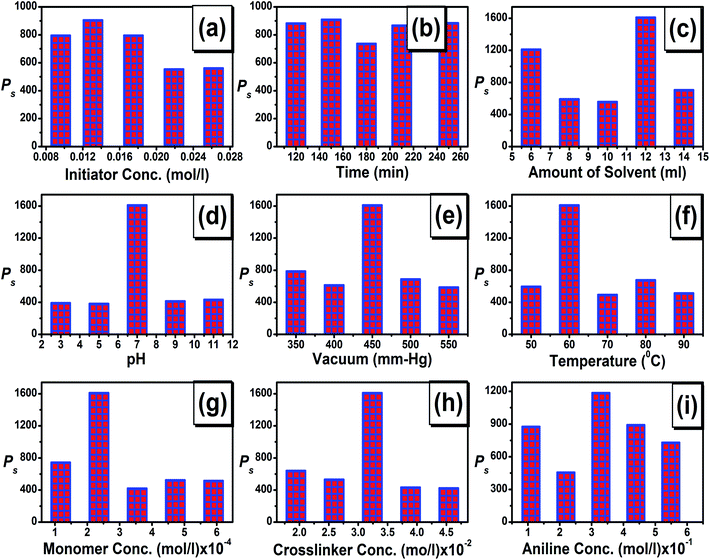
In the present investigation, we have optimized various reaction parameters (i.e. monomer, initiator and cross-linker concentration, amount of solvent, pH, reaction time, vacuum and reaction temperature) to obtain the maximum percentage swelling. The results are briefly discussed below. The effect of APS (initiator) concentration on the %S was studied and the results are shown in Fig. 1a. It was observed that the %S increases up to a certain level and reaches a maximum value at a concentration of 0.0131 mol l−1, beyond which it decreased rapidly. The enhancement in %S at a lower APS concentration may be attributed to the increase of macro radicals on the backbone.27 With an increase in initiator concentration, more free radicals attacked the gum ghatti backbone, which results in the generation of more radicals on the gum ghatti backbone.28 Thus, more active sites of gum ghatti could react with the MAA, which leads to the propagation of polymer chains. Subsequent decreases in the %S capacity at higher APS concentration can be attributed to excessive radicals leading to terminating steps, and hence decrease in the molecular weight of the grafted hydrogel.6,27,29The effect of reaction time was studied by changing the reaction time from 120 to 250 min while keeping the other reaction parameters constant, as shown in Fig. 1b. The %S increased initially with an increase in reaction time up to 150 min and thereafter decreased slightly. The decrease in the %S at higher reaction times was due to the reduction of the consuming reactants.4
Fig. 1c shows the variation of the %S of Gg-cl-poly(MAA) as a function of solvent concentration. It can be observed from the figure that %S first decreased up to 10 ml of solvent and then increased at 12 ml of solvent. A further increase in the amount of solvent resulted in a decreasing concentration of hydroxyl radicals and lead to the decrease in active sites and hence a decrease in %S.4
The %S of the Gg-cl-poly(MAA) hydrogel was studied at various pH's ranging from 3 to 11, and the results are shown in Fig. 1d. The maximum %S was found at a pH of 7, whereas it was found to be less in acidic and alkaline media. It has been reported that under acidic pH conditions, most of the carboxylate ion groups are protonated, thus the main anion–anion repulsive forces are very weak, which results in a reduced swelling capacity. The %S loss in alkaline solution is explained on the basis of the charge screening effect arising due to an excess of Na+ ions in the swelling medium. These ions shield the carboxylate ions and inhibit effective anion–anion repulsion between the different carboxylate groups; hence a lower %S was observed. Similar results have been observed in our previous work.4,22,23
The %S was studied at different vacuum pressures (350–550 mm Hg), and the results are shown in Fig. 1e. It was found that the %S was increased with an increase in the vacuum level but only to a certain extent. The maximum %S was found at a vacuum level of 450 mm Hg, and after that it was observed that the %S decreased.
The influence of reaction temperature on the %S was investigated from 50 to 90 °C and the results are shown in Fig. 1f. It was found that the maximum %S was observed at 60 °C and thereafter decreased with a rise of temperature up to 90 °C. The higher water absorbency at lower temperatures is explained on the basis of following points: (i) increasing the number of free radicals generated on the gum ghatti backbone, (ii) increased propagation of the graft copolymerization onto gum ghatti, (iii) improved diffusion of MAA and APS into and onto the gum ghatti, and (iv) enhancement in the mobility of the MAA molecules and their higher collision probability with gum ghatti macro radicals at a lower temperature.27–29 The observed behaviour at higher temperatures is attributed to the termination of the growing polymeric chains and to the occurrence of chain transfer reactions.28
In the swelling study, the monomer concentration was varied from 1.18 × 10−4–5.9 × 10−4 mol l−1 (Fig. 1g). As seen here, the hydrogel synthesized with lower monomer concentrations exhibited higher %S values. At lower monomer concentrations, the degree of dilution of the hydrogel is more, which causes an increase in the water content of the hydrogel and led to a high %S value. At a higher monomer concentration, %S decreased, which may be attributed to preferential homopolymerization over graft copolymerization and increase in viscosity of the medium, which hinders the movement of free radicals and monomer molecules.27
It is widely accepted that, when a cross-linker is added to a hydrogel, a decrease in the %S is achieved because the molecules of the cross-linker are placed between the chains of monomers.22 This leads to a reduction in the hydrophilic group members and %S. As shown in Fig. 1h, the maximum absorbency is found at lower MBA concentrations. At a higher cross-linker concentration, the cross-linking density increases, which results in the reduction of the network space and thereby less water enters the hydrogels.6
Optimization of aniline in acidic medium for the synthesis of Gg-cl-poly(MAA-IPN-aniline)
The effect of different aniline concentrations on the swelling capacity of graft copolymer has been studied while keeping the other reaction parameters constant (Fig. 1i). It has been observed that with an initial increase in aniline concentration, there was slight change in percentage swelling. However, after reaching the maxima, further increase in aniline concentration resulted in a decreased swelling capacity of the synthesized sample (Fig. 1h). This could be due to the increased compactness, decreased pore size and formation of the PANI homopolymer, which gives rise to decreased water absorbency.4,22,23Current–voltage characteristics
Fig. 2a shows the current–voltage (I–V) characteristics of the cross-linked IPN samples at room temperature. It is observed that the I–V characteristics of the IPN are completely symmetrical with respect to the polarity of the applied voltage. It has been found that the I–V characteristics were linear in nature and obey Ohm's law. This behaviour is explained by the conduction mechanism of conducting polymers (CPs). It has been reported in the literature that charge conduction in CPs is not only due to electron and holes such as in an intrinsic semiconductor but is also caused by the formation of polarons and bipolarons.4,22,23 On the other hand, it was noticed that the current of the cross-linked samples increased up to a 1.5 N HCl concentration and further increased the dopant concentration, which resulted in a reduction of the current. The improvement in the electrical conductivity with an increasing HCl concentration was due to an increasing degree of protonation of the imine group of PANI.4,22 The decrease in electrical conductivity at a higher HCl concentrations was attributed to the over protonation of the PANI chains, which causes a decrease in the delocalization length of PANI and hindrance in the amount of the electrons between the valence band and conduction band.4,22,23Thermal behaviour
The grafting was further supported by TGA (Fig. 2b). The gum ghatti and cross-linked hydrogels showed an initial mass loss up to 200 °C, which may be due to the removal of moisture, solvents, volatile components, and unreacted cross-linking agents or monomers.4,22,23 In the case of gum ghatti, a sharp mass loss of about 80–90% is observed between 207 and 536 °C. The observed mass loss is attributed to the loss of the hydroxyl group of gum ghatti as a water molecule or the decomposition of gum ghatti. The TGA curve of the Gg-cl-poly(MAA) shows a lower mass loss initially, but at the intermediate stage, it decreased continuously up to 465 °C. It was found that the percent residual mass of Gg-cl-poly(MAA) was higher than the gum ghatti at 465 °C, which reflects the cross-linking of MAA onto gum ghatti. However, at the later stage, it became almost constant, which may be due to the formation of a rigid cross-linked network, making it thermally more stable. Interestingly, the TGA spectrum of Gg-cl-poly(MAA-IPN-aniline) showed behaviour similar to that observed in the case of Gg-cl-poly(MAA). In the latter case, the percentage residual mass of Gg-cl-poly(MAA-IPN-aniline) was higher than Gg-cl-poly(MAA) and Gg at 465 °C. The results indicate that the introduction of PANI to the Gg-cl-poly(MAA) network resulted in an increase in thermal stability. Moreover, Singh et al.30 have reported that grafted gum is thermally more stable than native gum.X-ray diffraction studies
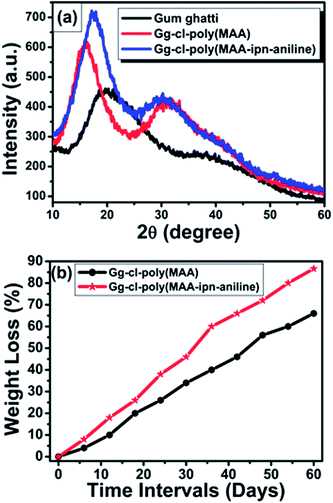
The XRD pattern of gum ghatti and the cross-linked hydrogels was recorded and are shown in Fig. 3a. A broad peak at 19.74° is characteristic of gum ghatti.4 The Gg-cl-poly(MAA) hydrogel showed a different XRD pattern than that from the gum ghatti, which confirms the formation of a cross-linked hydrogel. The MAA-modified gum ghatti shows an enhanced and slightly shifted main peak, which confirms that MAA reacts with the hydroxyl groups of the backbone. The overall increase in peak intensity suggests the convergence of the gum ghatti towards more ordered states after cross-linking; hence, the polymer became more semi-crystalline in nature. Furthermore, on moving from Gg-cl-poly (MAA) to Gg-cl-poly (MAA-IPN-aniline), the intensity of the diffraction peak also increases, which indicated the enhancement in crystallinity. This enhancement in crystallinity can be attributed to the interaction of the PANI chains in the cross-linked network of Gg-cl-poly(MAA).4,22,23 The PANI chains are cross-linked with each other during the polymerization process to form a network structure. This phenomenon relates the changes in the surface morphology revealed by the SEM images. Thus, the XRD patterns of the cross-linked hydrogels provide additional evidence of crosslinking onto the gum ghatti (Fig. 4). | ||
| Fig. 4 FTIR spectra of the cross-linked hydrogels before and after biodegradation (a) Gg-cl-poly(MAA) and (b) Gg-cl-poly(MAA-IPN-aniline). | ||
Biodegradation of Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline)
Biodegradability with variable degradation rates is much more important for most biomedical applications.31 The degradation was performed in a soil burial test at room temperature. It was reported that the time needed for complete loss of the hydrogels was used to assess their degradibility.32 The Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline) hydrogels were transparent and blackish in color with a smooth surface. Upon visual examination, the colour and surface of the hydrogels changed after the soil burial test, associated with microbial growth or morphological alterations. This clearly reflected the degradation nature of the cross-linked hydrogels in the soil burial conditions. This was supported by the FTIR and SEM analysis. The cross-linked hydrogels after 60 days of the soil burial test showed a distorted shape when compared to the original samples, which effected their mechanical properties.25 It has been widely accepted that the degradation of polymers depends on several factors, e.g. pH, oxygen content, temperature, availability of mineral nutrients and humidity, which are responsible for the growth of microorganisms.25 Fig. 3b shows the degradation profiles of the hydrogels buried in the moistened soil monitored after every 6 days. The percentage weight loss calculation was found to be an important method employed to check the biodegradation behaviour of the materials during the soil burial test. It also gives a degradation rate of each sample. As can be seen, the percentage weight loss of the hydrogels increased continuously with an increase in the number of days. It might be due to the dissociation of unreacted or low molecular weight (MW) macromers into the soil during the burial test. On the other hand, the soluble materials might leave some open pores through which water can penetrate easily. Thus, the percentage weight loss are different for Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline).The degradation rate of IPN in the soil burial test was faster than for the semi-IPN. The semi-IPN lost 66% weight in the soil burial test after 60 days. On the other hand, IPN samples were readily mineralized by the microorganisms, reaching a maximum degree of 86.6% after 60 days. Degradation of the semi-IPN and IPN samples under the soil burial conditions may be due to the breakage of large molecules into small molecules of low molecular weight under bacterial digestion or bacterial secretion. The percentage grafting plays an important role for the biodegradation of the cross-linked hydrogels. It was found that the semi-IPN has a high %G (Table 1) due to the breaking of covalent bonds, which takes more time and thereby results in less degradation as compared to the IPN hydrogel. It was mentioned earlier that aniline gets penetrated inside the semi-IPN network and the reaction conditions cause the formation and breaking of bonds. This leads to a lower %G as compared to the semi-IPN. Moreover, polyanline itself is not very stable and can get degraded in moistened conditions and leads to the breaking of bonds, as well as the formation of voids, thereby resulting in the easy attack of bacteria present in the soil. As a result, the increase in percentage weight loss of the IPN hydrogel might be occurring from some structural deformation and initial degradation of the molecular chains due to moisture and microorganisms in the soil.
Evidence of the biodegradability of the Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline) hydrogels
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carboxylic groups of gum ghatti, respectively. The peak at 1032 cm−1 corresponds to O–H bending vibrations. Another characteristic band of Gg was found at 1422 and 1032 cm−1 due to C–H bending and O–H bending vibrations, respectively.4,22 On comparing the IR spectra of gum ghatti and Gg-cl-poly(MAA) a variation in the intensity of the –OH stretching vibration was observed. The presence of the monomer (methacrylic acid) is also confirmed by the characteristic absorption bands at 1712 cm−1 and 2998 cm−1 due to the C
O stretching of the carboxylic groups of gum ghatti, respectively. The peak at 1032 cm−1 corresponds to O–H bending vibrations. Another characteristic band of Gg was found at 1422 and 1032 cm−1 due to C–H bending and O–H bending vibrations, respectively.4,22 On comparing the IR spectra of gum ghatti and Gg-cl-poly(MAA) a variation in the intensity of the –OH stretching vibration was observed. The presence of the monomer (methacrylic acid) is also confirmed by the characteristic absorption bands at 1712 cm−1 and 2998 cm−1 due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–H stretching vibrations respectively.23 The presence of additional bands at 796, 966, 1175, 1268, 1394, 1712, 2616 and 2998 cm−1 in the spectrum of Gg-cl-poly(MAA) indicate that the grafting has taken place on gum ghatti.24 These data are supportive of the grafting reaction between MAA and Gg. The FTIR studies of Gg-cl-poly(MAA) at different stages of biodegradation were carried out. It showed that at the early stage (stage-I) of the biodegradation (after 25 days) the peaks at 1175, 1258, 1492, 2589, 534, 1641, and 3597 cm−1 have almost disappeared. However, the transmittance of the peaks decreased and some of the peaks shifted due to the breakdown of the covalent bonds and cross-linking.12 In the final stage, i.e. stage-II (after 60 days), the individual compounds started degrading. It is believed that after 25 days the degradation of the gum ghatti and monomers was the major process. The FTIR spectrum of the second stage showed the peaks related to gum ghatti and methacrylic acid degradation when compared to the pristine and first stage.13 The degradation continued (up to 60 days) and most of the peaks related to the crosslinked hydrogels disappeared.
O and C–H stretching vibrations respectively.23 The presence of additional bands at 796, 966, 1175, 1268, 1394, 1712, 2616 and 2998 cm−1 in the spectrum of Gg-cl-poly(MAA) indicate that the grafting has taken place on gum ghatti.24 These data are supportive of the grafting reaction between MAA and Gg. The FTIR studies of Gg-cl-poly(MAA) at different stages of biodegradation were carried out. It showed that at the early stage (stage-I) of the biodegradation (after 25 days) the peaks at 1175, 1258, 1492, 2589, 534, 1641, and 3597 cm−1 have almost disappeared. However, the transmittance of the peaks decreased and some of the peaks shifted due to the breakdown of the covalent bonds and cross-linking.12 In the final stage, i.e. stage-II (after 60 days), the individual compounds started degrading. It is believed that after 25 days the degradation of the gum ghatti and monomers was the major process. The FTIR spectrum of the second stage showed the peaks related to gum ghatti and methacrylic acid degradation when compared to the pristine and first stage.13 The degradation continued (up to 60 days) and most of the peaks related to the crosslinked hydrogels disappeared.The FTIR spectrum of Gg-cl-poly(MAA-IPN-aniline) showed the characteristics peaks of PANI, as well as Gg-cl-poly(MAA).23 The bands at 1498 cm−1 and 1602 cm−1 represent the benzoid and quinoid ring vibrations of PANI, respectively. The band at 1260 cm−1 indicated the conducting form of PANI.33 The bands at 766 cm−1 and 2932 corresponded to an out-of-plane bending vibration of the C–H on the para-disubstituted rings.4,22 The band at 1706 cm−1 corresponded to the C–O stretching of the carboxylic acid group. The shifting and formation of new bands supported the formation of the Gg-cl-poly(MAA-aniline) hydrogel. The groups obtained for Gg-cl-poly(MAA-IPN-aniline) gives a strong proof for the cross-linking of aniline onto the Gg-cl-poly(MAA). The comparative IR spectra of the cross-linked hydrogels gives a clear visualization of the change in group frequencies that has occurred as a result of grafting. In the case of the first stage of degradation of Gg-cl-poly(MAA-IPN-aniline), the peak intensity of all the bands decreased drastically and some of the peaks disappeared. Moreover, the peak positions of the bands were also shifted, which indicates degradation of the cross-linked hydrogel as mentioned earlier. The FTIR spectra of stage-II (after 60 days) showed the peaks related to gum ghatti, acrylamide and polyaniline degradation. Interestingly, after 60 days of the soil burial degradation test, most of the peaks related to the cross-linked hydrogel have disappeared due to the complete breakdown of the samples (Fig. 6b).34
The change in the intensity of the peaks after the first stage of degradation may be due to the disintegration of the cross-linking by bacterial action.35 Most of the bonds are broken up by enzymes secreted by microbes and lead to by-products, which are reflected in the peaks shifting and the formation of new peaks.5 The hydrolysis and occurrence of new reactions causes shifts in the peak positions. In the second stage, we observed more degradation and weight loss because most of the peaks disappeared due to the breakdown of the vinyl monomer and rupture of the polyaniline chains from the backbone. Thus, it can be summarized from the above studies that the grafted chains of gum ghatti, methacrylic acid and aniline were degraded.5,13 However, the percentage of degradation was found to be more in Gg-cl-poly(MAA-IPN-aniline), which is in line with the percentage weight loss observed for cross-linked hydrogels.
 | ||
| Fig. 5 SEM images of gum ghatti and the Gg-cl-poly(MAA) cross-linked hydrogel before and after biodegradation. | ||
The SEM images of Gg-cl-poly(MAA-IPN-aniline) exhibits a cloudy sky-type surface morphology (Fig. 6a). These micrographs indicate that after the reaction with polyaniline the hydration and cross-linking varies inversely, which is also in line with the thermal analysis. The three micrographs clearly show the variation in the chemical structures and changes in surface morphology in the cross-linked networks. In the SEM images of Gg-cl-poly(MAA-IPN-aniline), during degradation stage-I (Fig. 6b), some cracks appeared due to the start of crosslink breakdown between different polymeric chains. However, the surface of Gg-cl-poly(MAA-IPN-aniline) during degradation stage-II (Fig. 6c) was completely fractured and porous. The enhanced degradation at this stage might be because of the complete breakdown of the covalent bonds between different polymeric chains through chemical and enzymatic degradation.5,13 From the above study, it can be suggested that the cross-linked hydrogels degraded by the fungal and bacterial species produced by the secretion of microorganisms during the soil burial test.5 Thus, it can be concluded from the morphological studies that the cross-linked networks of Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline) were degraded in the soil burial test. The cross-linked hydrogels are found to be biodegradable and can be utilized as a flocculent for the treatment of waste water.
Water retention properties of cross-linked hydrogels in different types of soil
Recently, great attention has been paid to the application of cross-linked hydrogels in agriculture, soil improvement and plant growth.36 Cross-linked hydrogel pieces distributed in soil are capable of absorbing water due to their large absorption capacity; consequently, they should be able to improve the water-holding capacity of the soil and promote optimal plant growth in drought-like conditions.37,38Taking this into account, the synthesized hydrogels have been investigated to study their water retention properties in different soil samples by noting down the weight of the samples everyday till a constant reading was obtained. The water evaporation ratio was calculated and the results are shown in Fig. 7. It was observed that the water evaporation ratio in the soil samples treated with the hydrogels investigated here was less than that of the soil without the hydrogels (control). In other words, the synthesized hydrogels can be effectively utilized in arid or drought-prone regions to transform them into “green and fertile lands.” As shown in Fig. 7, the content of water remaining in the different soil samples decreased with an increase in time, but the reduction rate was obviously different for each soil sample with and without the cross-linked hydrogels. Fig. 7a shows the water evaporation ratio of clay soil with and without the synthesized hydrogels. The clay soil mixed with cross-linked hydrogels exhibits a slower rate of water evaporation compared with the control hydrogel. The clay soil without hydrogels lost all of the absorbed water in 19 days, whereas the water evaporation period of the clay soil mixed with the semi-IPN hydrogel could be prolonged up to 31 days, while that of the clay soil with the IPN hydrogels was evaporated completely in 28 days. Fig. 7b shows the water evaporation ratio of the synthesized hydrogels in sand. In this case, with semi-IPN water evaporated in 23 days, whereas with IPN water evaporated in 18 days, while without hydrogels the water evaporated in 14 days. As expected in the case of a mixture of sand and soil the water evaporated between the 23rd and 31st day (Fig. 7c). Thus, Gg-cl-poly(MAA) had good water-retention capacities as compared to the Gg-cl-poly(MAA-ipn-aniline) hydrogel in clay soil, sand and a mixture of both; thus, it could be possible to save and manage water with cross-linked hydrogels. It has already been reported that the mean percentage swelling capacity of the Gg-cl-poly(MAA) (1602%) with 60% percentage grafting was higher than that of Gg-cl-poly(MAA-IPN-aniline) hydrogel with percentage grafting of 29%, having a percentage swelling of 1177% (Table 1). These results revealed that the synthesized hydrogels absorbed a large quantity of water in them, and allowed the absorbed water to be slowly released with a decrease in the moisture content of the soil samples. It has been reported that the enhancement in the water uptake capacity of the hydrogel reduced the irrigation requirement of many plants.39,40 Thus, it can be concluded that the synthesized hydrogels were effective in soil-moisture retention through the slow release of water molecules into the soil samples and can be used as an effective water-retaining device for agricultural and horticultural purposes.36,41
 | ||
| Fig. 7 The water retention behaviour of the synthesized hydrogels in (a) clay soil, (b) sand, and (c) clay soil + sand. | ||
Dye adsorption study of Gg-cl-poly(MAA-IPN-aniline)
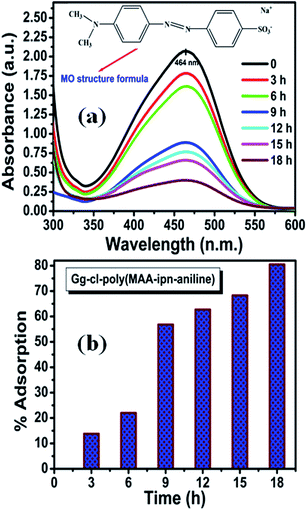
For adsorption of methyl orange (MO) dye, the Gg-cl-poly(MAA-IPN-aniline) hydrogel was placed in an aqueous solution of MO dye. MO is a well-known anionic dye, which has been widely used in textile, printing, paper, pharmaceutical and food industries.42 It has been reported in the literature that the main interactions between dyes and hydrogels are hydrophobic, electrostatic, hydrogen bonding and surface functional group interactions.43,44 The presence of reactive hydroxyl (–OH) and amino (–NH2) groups in Gg-cl-poly(MAA-IPN-aniline) makes it a potential adsorbent for dyes.4 Fig. 8a shows the adsorption behaviour of Gg-cl-poly(MAA-IPN-aniline) in a MO solution as a function of contact time. Note that the MO dye has an absorption band at 464 nm. The adsorption of the dye in the synthesized hydrogel network was monitored by UV-visible spectroscopy by observing the reduction in the absorbance value at 464 nm with time. Fig. 8a clearly shows that the adsorption capacity of MO increased with increasing contact time and that the adsorption process reached equilibrium after 18 h. It can also be seen from Fig. 8a that the absorbance intensity decreases with an increase in contact time. It is believed that the MO dye was adsorbed onto the Gg-cl-poly(MA-IPN-aniline) surface with increasing contact time due to the preferential adsorption of MO dye onto the synthesized IPN, which consists of –OH and –NH2 groups.4,45 As a result of this, a sharp decrease of absorbance was observed at the beginning, and a small reduction in the absorbance was observed after 9 h contact time until the adsorption process reached equilibrium. At the beginning, the rate of removal of MO was higher, which is due to the presence of the more active sites available for interaction with the dye molecules, and the adsorption sites on the Gg-cl-poly(MAA-IPN-aniline) surfaces are almost saturated by the MO dye molecules due to the decreased or lesser number of active sites available. Therefore, the adsorption process of the cross-linked hydrogel slows down due to the gradual enhancement of charge repulsive forces between the absorbed and unabsorbed dyes.45 In order to understand the dye adsorption in Gg-cl-poly(MAA-IPN-aniline), the % adsorption was calculated for the hydrogels and is shown in Fig. 8b. We can see that Gg-cl-poly(MAA-IPN-aniline) absorbed 80.5% dye in 18 h. The dye adsorption capacity of Gg-cl-poly(AA-IPN-aniline) is also explained in terms of strong π–π* interactions prevalent in the IPN hydrogel due to the conjugated nature of PANI, which are useful for effective charge transfer between the cross-linked hydrogels,46,4 and hence enhances the adsorption of the anionic MO dye onto the IPN surface. Since MO is an anionic dye, the electrostatic interaction between the –OH group of poly(methacrylic acid) and the –NH group of PANI in the Gg-cl-poly(MAA-IPN-aniline) hydrogel is expected to be dominant for the removal of dye.47 The suggested mechanism of binding the MO dye with the IPN hydrogel is shown in Scheme 2. It is believed that the MO dye and the Gg-cl-poly(MAA-IPN-aniline) network interact with each other via hydrogen bond formation. Moreover, the developments in polysaccharide-based materials used as adsorbents in waste water treatment have been reviewed in detail in a previous study.48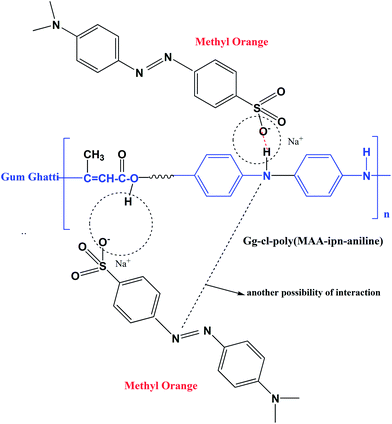 | ||
| Scheme 2 Schematic mechanism of the adsorption of MO dye onto the Gg-cl-poly(MAA-IPN-aniline) hydrogel. | ||
Conclusions
Synthesis of an interpenetrating network using methacrylic acid and aniline improved the property profile of the polymer and can be used in various technical fields. The Gg-cl-poly(MAA-IPN-aniline) hydrogel was observed to possess electrical conductivity. Both Gg-cl-poly(MAA) and Gg-cl-poly(MAA-IPN-aniline) were found to be degradable, which was further supported by FTIR and SEM studies. The percentage degradation of Gg-cl-poly(MAA-IPN-aniline) is higher than that of Gg-cl-poly(MAA) in the soil burial test. The synthesized hydrogels may be considered as effective alternatives to improve the moisture contents in different soils for agricultural use. The conducting IPN is a suitable material for the removal of MO dye from waste water.Acknowledgements
Our research was supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa. We thank the University of the Free State Cluster program for financial support. Authors are also thankful to department of Chemistry, University of the Free State for providing TGA spectra.References
- M. Shimao, Curr. Opin. Biotechnol., 2001, 12, 242–247 CrossRef CAS.
- H. Tian, Z. Tang, X. Zhuang, X. Chen and X. Jing, Prog. Polym. Sci., 2012, 37, 237 CrossRef CAS PubMed.
- B. D. Ulery, L. S. Nair and C. T. Laurencin, J. Polym. Sci., Part B: Polym. Phys., 2011, 49(12), 832 CrossRef CAS PubMed.
- K. Sharma, B. S. Kaith, V. Kumar, V. Kumar, S. Som, S. Kalia and H. C. Swart, RSC Adv., 2013, 3, 25830 RSC.
- H. Mittal, S. B. Mishra, A. K. Mishra, B. S. Kaith and R. Jindal, Int. J. Biol. Macromol., 2013, 58, 37 CrossRef CAS PubMed.
- G. Peng, S. Xu, Y. Peng, J. Wang and L. Zheng, Bioresour. Technol., 2008, 99, 444 CrossRef CAS PubMed.
- L. Wu, M. Liu and R. Liang, Bioresour. Technol., 2008, 99, 547 CrossRef CAS PubMed.
- S. Ghorai, A. Sinhamahpatra, A. Sarkar, A. B. Panda and S. Pal, Bioresour. Technol., 2012, 119, 181 CrossRef CAS PubMed.
- B. S Kaith, R. Jindal, H. Mittal and K. Kumar, J. Appl. Polym. Sci., 2012, 124, 2037 CrossRef.
- R. C. Mundargi, S. A. Patil and T. M. Aminabhavi, Carbohydr. Polym., 2007, 69, 130 CrossRef CAS PubMed.
- A. S. Deshmukh, C. M. Setty, A. M. Badiger and K. S. Muralikrishna, Carbohydr. Polym., 2012, 87, 980 CrossRef CAS PubMed.
- H. Mittal, E. Fosso-Kankeu, S. B. Mishra and A. K. Mishra, Int. J. Biol. Macromol., 2013, 62, 370 CrossRef CAS PubMed.
- H. Mittal, S. B. Mishra, A. K. Mishra, B. S. Kaith, R. Jindal and S. Kalia, Carbohydr. Polym., 2013, 98, 397 CrossRef CAS PubMed.
- P. Rani, G. Sen, S. Mishra and U. Jha, Carbohydr. Polym., 2012, 89, 275 CrossRef CAS PubMed.
- Q. W. Tang, J. H. Wu, J. M. Lin, S. J. Fan and D. Hu, Carbohydr. Polym., 2008, 74, 215 CrossRef CAS PubMed.
- Q. W. Tang, J. H. Wu, H. Sun, S. J. Fan, D. Hu and J. M. Lin, Carbohydr. Polym., 2008, 73, 473 CrossRef CAS PubMed.
- A. Tiwari, J. Polym. Res., 2008, 15, 337–342 CrossRef CAS.
- A. Tiwari, Y. Sharma, S. Hattori, D. Terada, A. K. Sharma, A. P. F. Turner and H. Kobayshi, Biopolymers, 2013, 99, 334 CrossRef CAS PubMed.
- A. Tiwari and V. Singh, eXPRESS Polym. Lett., 2007, 1, 308 CrossRef CAS.
- A. Tiwari and V. Singh, Carbohydr. Polym., 2008, 4, 427 CrossRef PubMed.
- A. Tiwari, J. Macromol. Sci., Part A: Pure Appl.Chem., 2007, 44(7), 735 CrossRef CAS.
- K. Sharma, B. S. Kaith, V. Kumar, S. Kalia, V. Kumar, S. Som and H. C. Swart, eXPRESS Polym. Lett., 2014, 8, 267 CrossRef.
- B. S. Kaith, K. Sharma, V. Kumar, S. Kalia and H. C. Swart, Synth. Met., 2014, 187, 61 CrossRef CAS PubMed.
- K. Sharma, B. S. Kaith, V. Kumar, V. Kumar, S. Kalia, B. K. Kapur and H. C. Swart, Radiat. Phys. Chem., 2014, 97, 253 CrossRef CAS PubMed.
- W. Phetwarotai, P. Potiyaraj and D. Aht-Ong, J. Polym. Environ., 2013, 21, 95 CrossRef CAS.
- V. Singh, A. Tiwari and R. Sanghi, J. Appl. Polym. Sci., 2005, 98, 1652 CrossRef CAS.
- A. Pourjavadi, A. M. Harzandi and H. Hosseinzadeh, Eur. Polym. J., 2004, 40, 1363 CrossRef CAS PubMed.
- T. Sun, P. Xu, Q. Liu, J. Xue and W. Xie, Eur. Polym. J., 2003, 39, 189 CrossRef CAS.
- J. Chen and Y. Zhao, J. Appl. Polym. Sci., 2000, 75, 808 CrossRef CAS.
- V. Singh, A. Srivastava and A. Tiwari, Int. J. Biol. Macromol., 2009, 45, 293–297 CrossRef CAS PubMed.
- A. Wang, W. Zhu, Y. Zhang, Z. Yang and J. Ding, React. Funct. Polym., 2006, 66, 509 CrossRef PubMed.
- A. Tiwari, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, Acta Biomater., 2009, 5, 3441 CrossRef CAS PubMed.
- Y. Sharma, A. Tiwari, S. Hattori, D. Terada, A. K. Sharma, M. Ramalingam and H. Kobayashi, Int. J. Biol. Macromol., 2012, 51, 627 CrossRef CAS PubMed.
- M. Maiti, B. S. Kaith, R. Jindal and A. K. Jana, Polym. Degrad. Stab., 2010, 95, 1694 CrossRef CAS PubMed.
- B. S. Kaith, R. Jindal, A. K. Jana and M. Maiti, Bioresour. Technol., 2010, 101, 6843 CrossRef CAS PubMed.
- Saruchi, B. S. Kaith, R. Jindal and G. S. Kapur, Iran. Polym. J., 2013, 22, 561 CrossRef CAS PubMed.
- E. Karadag, D. Saraydin, Y. Caldiran and O. Guven, Polym. Adv. Technol., 2000, 11, 59 CrossRef CAS.
- K. S. Kazanskji and S. A. Dubrovskii, Adv. Polym. Sci., 1992, 104, 97 CrossRef.
- S. A. Shahid, A. A. Qidwai, F. Anwar, I. Ullah and U. Rashid, Molecules, 2012, 17, 9397 CrossRef CAS PubMed.
- H. A. Abd El-Rehim, A. H. El-Sayed and H. L. Abd El-Mohdy, J. Appl. Polym. Sci., 2004, 93, 1360 CrossRef CAS.
- K. S. Soppimath, T. M. Aminabhavi, A. R. Kulkarni and W. E. Rudzinsk, J. Controlled Release, 2001, 70, 1 CrossRef CAS.
- J. Ma, F. Yu, L. Zhou, L. Jin, M. Yang, J. Luan, Y. Tang, H. Fan, Z. Yuan and J. Chen, ACS Appl. Mater. Interfaces, 2010, 4, 5749 Search PubMed.
- Z. Wu, H. Joo and K. Lee, Chem. Eng. J., 2005, 112, 227 CrossRef CAS PubMed.
- Z. Wu, L. You, H. Xiang and Y. Jiang, J. Colloid Interface Sci., 2006, 303, 346 CrossRef CAS PubMed.
- Y. Zeng, L. Zhao, W. Wu, G. Lu, F. Xu, Y. Tong, W. Liu and J. Du, J. Appl. Polym. Sci., 2013, 127, 2475 CrossRef CAS.
- D. F. Acevedo, J. Balach, C. R. Rivarola, M. C. Miras and C. A. Barbero, Faraday Discuss., 2005, 131, 235 RSC.
- S. Zaremotlag and A. Hezarkhani, Environ. Earth Sci., 2014, 71, 2999 CrossRef PubMed.
- G. Crini, Prog. Polym. Sci., 2005, 30, 38 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |