Simulation of conformational properties of end-grafted diblock copolymers
Hong
Li
ab,
Bin
Gong
a,
Chang-Ji
Qian
*b,
Chao-Yang
Li
c,
Jian-Hua
Huang
d and
Meng-Bo
Luo
e
aShool of Computer Science and Technology, Shandong University, Jinan 250100, China
bDepartment of Physics, Wenzhou University, Wenzhou 325035, China. E-mail: qcj@wzu.edu.cn
cDepartment of Physics, Hangzhou Normal University, Hangzhou 310036, China
dDepartment of Chemistry, Zhejiang Sci-Tech University, Hangzhou 310018, China
eDepartment of Physics, Zhejiang University, Hangzhou 310027, China
First published on 10th June 2014
Abstract
Conformational properties of end-grafted flexible diblock copolymers were studied by using Monte Carlo simulation. The copolymers, XAnBn and XBnAn, are grafted with the end X to a flat surface which attracts monomers A but repulses monomers B. Results show that the blocks A in XAnBn and XBnAn are adsorbed upon the surface at low temperature, and the adsorption of blocks A takes place roughly at the same temperature. However, the conformational size and instantaneous shape of the blocks A and B in XAnBn and XBnAn are different. The possible reasons were discussed and they were interpreted by different properties and grafting locations of the two blocks.
1. Introduction
Adsorption of polymers on attractive surfaces or interfaces plays an important role in various areas of technological applications and biological processes.1–6 The conformation of adsorbed polymers can be classified into distinct types according to the adsorption strength.7,8 It is relevant to many applications, such as gel permeation chromatography,1,2 compatibilization by copolymer,3,4 copolymer separation,5 medical implants and bioaffinity sensors.6 The behavior of polymers near an attractive surface has been numerous studied by theory and computer simulation.8–11The adsorption of end-grafted homogeneous polymers on a flat attracting surface was intensively investigated.12–17 Every monomer contacting with the surface is assigned an attraction energy E. There is a phase transition from a desorbed state at high temperatures to an adsorbed state at low temperatures beyond a critical value Tc, which is named as the critical adsorption point (CAP). The conformation of an adsorbed polymer changes from three dimensional to a quasi two dimensional conformation at CAP. The properties of adsorption, conformational size and shape of the polymer show different behaviors at the temperature below, above, and near the CAP. At CAP, it was found that the conformational sizes, such as mean square end-to-end distance 〈R2〉 and mean square radius of gyration 〈RG2〉, reach minimum.16 But it is difficult to directly measure the conformational properties of a single polymer.18 Theoretical study of a single polymer chain is important as it provides a tool for understanding the properties of polymer aggregates.
The critical adsorption and conformational properties of end-grafted AB copolymers has also been investigated.19–21 The critical adsorption point, statistical conformational size and shape of the copolymer are the key factors to understand the behavior of copolymer, since these properties are dependent on the chain length and polymer-surface attraction.19–21 The AB copolymer is composed of two types of monomers A and B that have different properties. Therefore AB copolymer can be modeled as a synthetic protein or DNA.22 The properties of copolymer tethered to surface are more complex. The copolymer is usually modeled as having attractive monomers A and non-attractive monomers B. It was found that copolymer's properties depend not only on the whole chain length but also on the block size of the attractive monomers and the interaction strength.20,21 Copolymers tend to adsorb with the attractive blocks rather flat on the surface, whereas the non-attractive block in one dangling tail (or loop) protrudes into the solution.19 Besides, for end-grafted diblock copolymer AnBn, the adsorption and conformational properties will be also dependent on the position of non-attractive block B, i.e. on the grafted end which is near B or near A. Study the adsorption of end-grafted diblock copolymers is helpful to understand the macroscopic behaviors and structures of block-copolymer on surface.23–25
In the present paper, we study the adsorption of two simple flexible diblock copolymers XAnBn and XBnAn with the end monomer X grafted to a flat surface. The conformational size and instantaneous shape of the whole copolymer and that of the blocks A or B in XAnBn and XBnAn are studied. We find that the adsorption property of the blocks A in both copolymers is similar, but the conformational properties are quite different for the block A and block B. As a result, properties of diblock copolymer XAnBn are different from XBnAn. Our results show that the conformational properties of the whole copolymer as well as that of each block are dependent on the position of the non-attractive block B.
2. Model and calculation method
We use self-avoiding walk (SAW) copolymers on the simple cubic lattice with one end grafted to a flat surface. The flat surface located at z = 0 is assumed infinitely large and impenetrable to copolymer chain. The copolymer XAnBn or XBnAn has equal block length for the blocks A and B. The polymer length is defined as N = 2n. The graft monomer X is fixed at position (0, 0, 1), and copolymer is restricted to lie in the upper half space (z > 0). Each monomer occupies one site of the lattice. The bond length in copolymer can fluctuate from 1, and
and 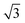 lattice unit.26–28 We consider that two types of monomers are different in the interaction with the surface. The interaction between the monomer A and the surface is attractive, whereas that between the monomer B and the surface is pure repulsive. Especially, every monomer locating at layer z = 1, namely contacting with the surface, is assigned an interaction energy EAS = −1 for monomer A and EBS = 0 for monomer B. We consider the case that the interaction between two non-bonded monomers are pure repulsive and is assigned as EAA = EBB = EAB = 0 in the simulation. Here, we only consider a simple flat surface with short-range interactions, which is widely used in theory and simulation.11–17 Whereas long-range interactions, such as electrostatic interactions which might be exist in real surface, are ignored in the present work.
lattice unit.26–28 We consider that two types of monomers are different in the interaction with the surface. The interaction between the monomer A and the surface is attractive, whereas that between the monomer B and the surface is pure repulsive. Especially, every monomer locating at layer z = 1, namely contacting with the surface, is assigned an interaction energy EAS = −1 for monomer A and EBS = 0 for monomer B. We consider the case that the interaction between two non-bonded monomers are pure repulsive and is assigned as EAA = EBB = EAB = 0 in the simulation. Here, we only consider a simple flat surface with short-range interactions, which is widely used in theory and simulation.11–17 Whereas long-range interactions, such as electrostatic interactions which might be exist in real surface, are ignored in the present work.
Fluctuation of bond in polymer is due to random Brownian motion resulted from random collisions between monomers and solvent molecules. In the dynamic model, a monomer is chosen randomly and attempts to jump one lattice spacing selected randomly from one of the six directions. This jump will be accepted if the following five conditions are satisfied: (1) the new site locates at z > 0, (2) the new bond vector still belong to the allowed bond set, (3) self-avoidance is obeyed, (4) two bonds do not intersect, and (5) the Boltzmann factor exp(−ΔE/kBT) > p, where 0 < p < 1 is a random number in (0, 1) and ΔE is the energy shift due to the change of monomer sites. The Boltzmann constant kB is set unity in the simulation. In one Monte Carlo step (MCS) all monomers except the grafted end in the chain attempt to move once.
Annealing method is adopted to simulate the temperature dependence of polymer properties. Starting at a high temperature T = 8, we slowly decrease T with a small step ΔT. The step is chosen as small as 0.05 near Tc, while a slightly big value is chosen away from Tc. The chain changed its configurations with time. At each T, the copolymer is first equilibrated for about 2.5N2.13MCS.15 Then we record the conformations of polymer at every 0.1N2.13MCS in the next 100 N2.13MCS. The results are averaged over 1000 conformations for one independent run. And our final results are further averaged over 1000 independent runs. The simulation codes are written in Fortran 90 with MPI.
In this work we have simulated the adsorption and conformation properties of copolymer AnBn with different grafted points and with different block length n varied from n = 5 to n = 200. We find that the conformational properties of three representative copolymers A50B50, A100B100, and A200B200 present the similar behaviors. However the difference in the conformational properties between XAnBn and XBnAn increases slightly with the increase in the block length n. Therefore, in this work we take XA200B200 and XB200A200 as the main samples.
3. Results and discussions
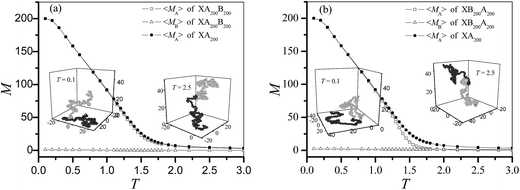
Fig. 1 shows the mean number of surface contacts for blocks A and B at different temperatures for the diblock copolymers XA200B200 and XB200A200. The block B almost does not contact with the surface since EB = 0, whereas the block A begins to be adsorbed at about T = 1.5 which is close to the CAP of homogeneous polymer.17 It means that the CAPs of XA200B200 and XB200A200 are about T = 1.5 and the non-attractive block B has little influence on the CAP of diblock copolymers AnBn. The difference between XA200B200 and XA200 is smaller than that between XB200A200 and XA200, indicating that the influence of the end-grafted block B is slightly more obvious than the dangled block B. At T > 1.7, the block A in XB200A200 does not contact the surface anymore. The insets show the snapshots of polymer configurations at high temperature T = 2.5 and at low temperature T = 0.1. The configurations of XA200B200 and XB200A200 at T = 2.5 are similar. Both copolymers fully extend in the solution. But at T = 0.1, large difference can be found for the block B: block B in XA200B200 behaves like an end-grafted chain whereas it in XB200A200 behaves like a ring with two end monomers B contacting with the surface. The block B stretches in the solution for XA200B200, whereas the middle part extends in solution for XB200A200. At T = 0.1, the contact number is M = 200 for both copolymers, indicating that all the monomers A lie on the surface and thus the conformation of block A is two-dimensional.The square end-to-end distances R2 and the square radius of gyration RG2 of a chain with length N are defined as:
 | (1) |
 | (2) |
![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) i and
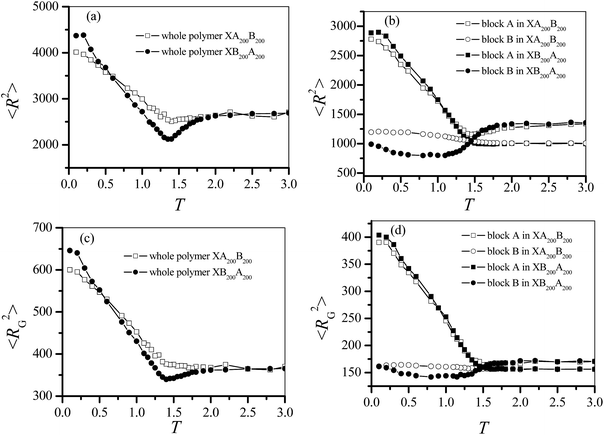
i and  are the position vectors of the ith monomer and the center of mass of the chain, respectively. The mean square end-to-end distances 〈R2〉 and the mean square radius of gyration 〈RG2〉 are calculated at different temperatures. At the same time, the mean square end-to-end distances and the mean square radius of gyration for the block A (〈RA2〉 and 〈RGA2〉) and the block B (〈RB2〉 and 〈RGB2〉) are also calculated. The results are presented in Fig. 2 for XA200B200 and XB200A200. The location T = 1.5 at which both 〈RA2〉 and 〈RGA2〉 are minima is defined as the CAP of the copolymer XA200B200 as the method adopted for homogeneous polymers.16 We find that both 〈R2〉s and 〈RG2〉s of XA200B200 and XB200A200 show the same behaviors. And difference between the two copolymers is only at low temperatures below CAP. The behavior of polymer conformation can be understood from the behaviors of blocks A and B.
are the position vectors of the ith monomer and the center of mass of the chain, respectively. The mean square end-to-end distances 〈R2〉 and the mean square radius of gyration 〈RG2〉 are calculated at different temperatures. At the same time, the mean square end-to-end distances and the mean square radius of gyration for the block A (〈RA2〉 and 〈RGA2〉) and the block B (〈RB2〉 and 〈RGB2〉) are also calculated. The results are presented in Fig. 2 for XA200B200 and XB200A200. The location T = 1.5 at which both 〈RA2〉 and 〈RGA2〉 are minima is defined as the CAP of the copolymer XA200B200 as the method adopted for homogeneous polymers.16 We find that both 〈R2〉s and 〈RG2〉s of XA200B200 and XB200A200 show the same behaviors. And difference between the two copolymers is only at low temperatures below CAP. The behavior of polymer conformation can be understood from the behaviors of blocks A and B.
〈RA2〉 of XA200B200 decreases with the decrease in T at high temperatures but increases with the decrease in T at low temperatures, which is consistent with the behavior of a homogeneous polymer.17 That implies that the dangled block B has little influence on the properties of XA200B200. 〈RA2〉 of XB200A200 however increases monotonically with the decrease in T. Such a difference results from that the block A of XA200B200 is initially end-grafted whereas the block A of XB200A200 is initially roughly free in the solution. At high temperature, 〈RA2〉 of XA200B200 is bigger than 〈RA2〉 of XB200A200. On the other hand, with the decrease of T, the block B of XA200B200 changes from a free conformation with small 〈RB2〉 at high T to an end-grafted conformation with a slightly larger 〈RB2〉 at low T. 〈RB2〉 of XA200B200 at T = 0.1 is roughly the same as 〈RA2〉 of XA200B200 at high temperatures. While the block B of XB200A200 changes from an end-grafted conformation at high T to a double end-grafted conformation at low T. We find 〈RB2〉 of XB200A200 changes nonmonotonically with T. A minimum of 〈RB2〉 is found when the polymer changes from an end-grafted conformation to a double end-grafted conformation. The properties of 〈RG2〉 are similar to those of 〈R2〉 for these two end-grafted diblock copolymers AnBn. The different conformational properties of block A or block B are due to their different surface interactions and their different positions in chain.
The dependence of 〈R2〉 and 〈RG2〉 on the chain length N of homogenous polymer is in scaling laws as 〈R2〉 ∝ N2ν and 〈RG2〉 ∝ N2ν.16,26,27 Here the exponent ν is dependent on polymer model and space dimension.29–31 For SAW chain, ν = 0.588 for a three-dimensional (3D) chain and 0.75 for two-dimensional (2D) one, respectively.29 While in a melt of poly(ethylene terephthalate) (PET), ν is dependent on chain length.30,31 It was found that the exponent changes from about 0.6 for short chains to about 0.5 for longer chain.30,31 We here examine whether the scaling laws are still valid for the end-grafted diblock copolymer chain. We calculate 〈R2〉 and 〈RG2〉 of copolymers XAnBn and XBnAn for different block lengths n = 5, 10, 20, 25, 40, 50, 75, 100, 150, and 200. We find that the scaling relation is still applicable for the copolymers XAnBn and XBnAn at different temperatures above, below or near CAP. For example, the dependence of 〈R2〉 and 〈RG2〉 on N in is presented in Fig. 3a for copolymers at low temperature T = 0.5 and at T = 1.4 near CAP. The dependence of the scaling exponent ν on the temperature T is plotted in Fig. 3b for the scaling law of 〈RG2〉 ∝ N2ν. We find that there is a transition for the value of ν with the decrease of T. The location of the transition point is near CAP. At the temperature T above CAP, the value of ν is close to the Flory exponent 0.588 of 3D SAW chain. At T below CAP, the value of ν increases with the decrease of T. At the temperature T = 0.5 or below, the scaling exponent ν = 0.675 is located between ν = 0.6 of three dimension and ν = 0.75 of two dimension, which is due to two dimensional conformation of block A adsorbed on the surface and three dimensional conformation of block B dangled in the solution. At T near CAP, the value ν of XBnAn is slightly smaller than that of XAnBn because the block B changes from an end-grafted conformation to a double end-grafted one. We find that the scaling exponent ν of 〈R2〉 ∝ N2ν is also similar to that of 〈RG2〉∝ N2ν.
The instantaneous shape of polymer can be expressed by the mean asphericity parameter 〈A〉 which is defined as
 | (3) |
 | (4) |
Fig. 4 shows the dependence of 〈A〉 on T for copolymers XA200B200 and XB200A200. Values of the mean asphericity parameter 〈A〉 are calculated for the whole copolymer 〈A〉, block A 〈AA〉 and block B 〈AB〉, respectively. We find that the variation of 〈A〉 is similar to that of 〈R2〉 and 〈RG2〉, indicating that there are correlations between shape and size for each block in the copolymers as that found in homogeneous polymer.33 〈AA〉 of the blocks A increases fast below T = 1.5, indicating the transition from 3D to 2D takes place roughly at the same temperature for both copolymers. The value of 〈AA〉 is about 0.62 at low temperatures, indicating that the 2D conformation of block A is elliptical. The increase of 〈AB〉 with decreasing temperature for the block B of XA200B200 shows that the block B changes to end-grafted conformation at low temperature. Whereas for the block B in XB200A200, it changes from an end-grafted conformation to a double end-grafted one. We find that 〈AB〉 decreases at first and then increases with the decrease in temperature.
 | ||
| Fig. 4 The mean asphericity parameter 〈A〉 at different temperatures T for whole copolymer chain (a) and for the block A and the block B (b) in copolymers XA200B200 and XB200A200. | ||
4. Conclusion
Dynamic Monte Carlo methods are carried out for the investigation of adsorption and conformational properties of diblock copolymers XAnBn and XBnAn. The conformational properties, the mean square end-to-end distance 〈R2〉, the mean square radius of gyration 〈RG2〉 and the mean asphericity parameter 〈A〉, are dependent on temperature and the property of block. We found that at high temperature (above CAP), the conformations of the whole diblock copolymer XAnBn and XBnAn are almost the same, whereas that of block A or block B in XAnBn and XBnAn exist difference due to the different positions of A and B in the end-grafted polymer. At low temperature (below CAP), the block A is attracted to the surface, whereas the block B in XAnBn is an end-grafted coil and that in XBnAn is a loop with two ends contacted with the surface. At the intermediate temperature (near CAP), the conformational size and instantaneous shape of the whole copolymer reach a minimum, especially in XBnAn. Our results show that one could distinguish different types of blocks in copolymer by measuring their temperature dependence of size and shape.Our results show that the difference in conformational properties of XAnBn and XBnAn is due to the different properties between two blocks and different positions of B. Therefore the difference will be reduced if the difference between two blocks is reduced. The cases of different block length between block A and block B are also considered for diblock copolymers XAmBn and XBnAm. However, the effect of block length A on the behavior of block B is quite interesting. If the block B is the dangled block as in XAmBn, the behavior of block B is roughly independent of the length of block A. Whereas if the block B is the end-grafted block as in XBnAm, the behavior of block B is strongly dependent on the length of block A. In this case, the block B changes from one end grafted chain at high temperature to a loop with two ends grafted. The behavior can be also observed but the transition temperature (close to CAP) is dependent on the length of block A, since CAP increases with the length of block A.8 Therefore, the general trend is that the effect of block A is reduced when the length of block A is decreased.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos. 11304231, 11374255, and 21171145 and by the Zhejiang Natural Science Foundation of China under Grant No. LY14B040004.References
- E. Uliyanchenko, S. Wal and P. J. Schoenmakers, Polym. Chem., 2012, 3, 2313–2335 RSC.
- B. Trathnigg, Prog. Polym. Sci., 1995, 20, 615–650 CrossRef CAS.
- N. Zgheib, J. L. Putaux, A. Thill, E. Bourgeat-Lami, F. D'Agosto and M. Lansalot, Polym. Chem., 2013, 4, 607–614 RSC.
- G. Pu, Y. Luo, A. Wang and B. Li, Macromolecules, 2011, 44, 2934–2943 CrossRef CAS.
- Y. S. Lipatov, A. E. Nesterov, T. D. Ignatova and D. A. Nesterov, Polymer, 2002, 43, 875–880 CrossRef CAS.
- L. A. Ruiz-Taylor, T. L. Martin, F. G. Zaugg, K. Witte, P. Indermuhle, S. Nock and P. Wagner, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 852–857 CrossRef CAS PubMed.
- R. J. Roe, J. Chem. Phys., 1965, 43, 1591–1598 CrossRef CAS PubMed.
- E. Eisenriegler, K. Kremer and K. Binder, J. Chem. Phys., 1982, 77, 6296–6320 CrossRef CAS PubMed.
- F. Wang and D. P. Landau, Phys. Rev. Lett., 2001, 86, 2050–2053 CrossRef CAS.
- D. P. Landau and F. Wang, Comput. Phys. Commun., 2002, 147, 674–677 CrossRef CAS.
- D. T. Seaton, T. Wüst and D. P. Landau, Comput. Phys. Commun., 2009, 180, 587–589 CrossRef CAS PubMed.
- H. Zhang, J. Zhu, J. He, F. Qiu, H. Zhang, Y. Yang, H. Lee and T. Chang, Polym. Chem., 2013, 4, 830–839 RSC.
- R. Decase, J. U. Sommer and A. Blumen, J. Chem. Phys., 2004, 120, 8831–8840 CrossRef PubMed.
- E. J. J. van Rensburg and A. R. Rechnitzer, J. Phys. A: Math. Gen., 2004, 37, 6875–6898 CrossRef.
- M. B. Luo, J. Chem. Phys., 2008, 128, 044912 CrossRef PubMed.
- H. Li, C. J. Qian and M. B. Luo, Polymer J., 2010, 42, 383–385 CrossRef CAS.
- H. Li, C. J. Qian, L. Z. Sun and M. B. Luo, J. Appl. Polym. Sci., 2012, 124, 282–287 CrossRef CAS.
- M. Vacha and S. Habuchi, NPG Asia Mater., 2010, 2, 134–142 CrossRef.
- O. A. Evers, J. M. H. M. Scheutjens and G. J. Fleer, Macromolecules, 1990, 23, 5221–5233 CrossRef CAS.
- Y. Tan, L. Fang, J. Xiao, Y. Song and Q. Zheng, Polym. Chem., 2013, 4, 2939–2944 RSC.
- S. Bhattacharya, H.-P. Hsu, A. Milchev, V. G. Rostiashvili and T. A. Vilgis, Macromolecules, 2008, 41, 2920–2930 CrossRef CAS.
- A. Meller, L. Nivon, E. Brandin, J. Golovchenko and D. Branton, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 1079–1084 CrossRef CAS.
- G. Srinivas, D. E. Discher and M. L. Klein, Nat. Mater., 2004, 3, 638–644 CrossRef CAS PubMed.
- S. Egli, H. Schlaad, N. Bruns and W. Meier, Polymers, 2011, 3, 252–280 CrossRef CAS PubMed.
- J. N. L. Albert and T. H. Epps III, Mater. Today, 2010, 13, 24–33 CrossRef CAS.
- J. S. Shaffer, J. Chem. Phys., 1994, 101, 4205–4213 CrossRef CAS PubMed.
- H. Li, C. J. Qian, C. Wang and M. B. Luo, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 012602 CrossRef.
- H. Li, C. J. Qian, C. Wang and M. B. Luo, Polym. Polym. Compos., 2012, 20, 107–110 CAS.
- A. Yethiraj, Macromolecules, 2003, 36, 5854–5862 CrossRef CAS.
- Q. Wang, D. J. Keffer, S. Petrovan and J. B. Thomas, J. Phys. Chem. B, 2010, 114, 786–795 CrossRef CAS PubMed.
- Q. Wang, D. J. Keffer, D. M. Nicholson and J. B. Thomas, Macromolecules, 2010, 43, 10722–10734 CrossRef CAS.
- M. B. Luo and J. H. Huang, J. Chem. Phys., 2003, 119, 2439–2443 CrossRef CAS PubMed.
- M. B. Luo, J. H. Huang, Y. C. Chen and J. M. Xu, Eur. Polym. J., 2001, 37, 1587–1590 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |