Polyimide as anode electrode material for rechargeable sodium batteries
Long Chen,
Wangyu Li,
Yonggang Wang,
Congxiao Wang and
Yongyao Xia*
Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and, Innovative Materials, Institute of New Energy, Fudan University, Shanghai 200433, China. E-mail: yyxia@fudan.edu.cn
First published on 28th May 2014
Abstract
A polyimide synthesized from 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) and ethylene diamine (EDA) was evaluated as a new anode material for sodium ion batteries (SIB). The polyimide delivers a discharge specific capacity of 140 mA h g−1 at an average potential of 2 V vs. Na+/Na with an initial coulombic efficiency of 97.6% and exhibits an excellent cycleability with a capacity retention of 90% over 500 cycles. A full SIB with polyimide anode and Na3V2(PO4)3/C cathode and Na4Fe(CN)6/C cathode was proposed.
1. Introduction
Lithium-ion batteries have been investigated extensively in the past few decades and used widely in many aspects of our society.1 However, the large-scale production of lithium-ion batteries for vehicle applications will eventually drive the cost of lithium higher in the near future. With this background, room-temperature sodium-ion batteries with abundant sodium resources and potentially low cost have been reconsidered particularly for such large-scale applications. While a number of promising cathode materials such as Na3V2(PO4)3,2 Na2FePO4F,3 Na4Mn9O18,4 Na4Fe(CN)6/C,5 NaCrO2 (ref. 6) have already been invented, the anode remains the main obstacle impeding the application of this system. In earlier development, the anodic materials used were mostly hard carbon materials.7,8 Recently, Qian et al. reported a Sb/C nanocomposite9 and amorphous phosphorus10 as anode materials for sodium ion batteries. Hu et al.11 reported that spinel Li4Ti5O12 can also store Na ions. However, their long term cycling stability and initial coulombic efficiency are much inferior to those in lithium ion batteries.Organic materials have recently attracted much more attention as alternative active materials for rechargeable Li-ion batteries.12–14 Aside from abundant resources, organic electrode materials also have several advantages of chemical diversity, tunable redox property, mechanical flexibility and possible high energy density, offering a wide selection for battery applications. However, a few organic electrode materials for Na-ion batteries were reported, but exhibit poor electrochemical performance such as limited cyclic ability and poor initial coulombic efficiency.15–18 In our opinion, dissolution of organic electrode materials in the electrolyte is the main reason for these problems, and the polymer has a better insolubility than small molecules.19 Recently Deng et al. reported the poly(anthraquinonyl sulfide) (PAQS) as anode material for Na-ion battery with an excellent cycleability.20 Based on this, polyimide is proposed herein for its stable and inactive framework which can avoid the unwanted dissolution. Its application as an electrode material for Li-ion batteries has been mentioned,21 further more it was also served as anode materials in aqueous LIB and SIB.22
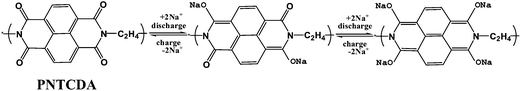
In this paper, we prepared 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA)-derived polyimide (i.e. PNTCDA) which was introduced as a novel electrode material for Na-ion batteries. When PNTCDA is used as the anode material, its reduction and oxidation are accompanied by the association and disassociation of Na+ ions with oxygen. Ideally, each formula unit is able to transfer four electrons through two steps (Scheme 1), which may allow a high theoretical specific capacity. The similar mechanism of lithium ion battery was mentioned before.21 Furthermore, the as-prepared PNTCDA anode was coupled with Na3V2(PO4)3/C and Na4Fe(CN)6/C cathode, respectively, to form 1.2 V full sodium ion batteries.
2. Experimental section
2.1 Material synthesis
PNTCDA was synthesized from 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) and ethylene diamine (EDA).21,23 Equimolar NTCDA and EDA reacted under reflux in the solvent of N-methylpyrrolidone (NMP) for 6 hours. The product was filtrated, washed with ethanol and NMP for several times, dried at 120 °C in air for 12 hours, then heated in nitrogen atmosphere for 8 hours at 300 °C.The Na3V2(PO4)3/C samples were synthesized by a sol–gel method. An aqueous precursor containing stoichiometric Na2HPO4·12H2O, NH4H2PO4, NH4VO3 and sucrose was stirred at a constant temperature of 90 °C until the water was evaporated. Then, the resulting deposit was placed in a porcelain boat and heated at 800 °C for 10 h under N2 flow in a tube furnace to form Na3V2(PO4)3/C product.
Na4Fe(CN)6/C composite was prepared, according to a previous report,5 by ball-milling dehydrated Na4Fe(CN)6 powder with Super P carbon in a mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for 1 h.
4 for 1 h.
2.2 Material characterization and electrochemical measurements
The as-prepared PNTCDA was characterized by Fourier transform infrared spectroscopy (FTIR) measurements through a NICOLET 6700 FI-TR Spectrometer using KBr pellet. The PNTCDA-based composite electrodes were prepared by mixing active materials, carbon black, and binder (polyvinyl difluoride) (PVDF) in N-methylpyrrolidinone (NMP) at a weight ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Subsequently, the slurry was cast uniformly on aluminum foil. The electrode film was vacuum-dried at 80 °C for 10 h to remove the solvent before roll-pressing. The electrode film was then punched into discs with diameters of 12 mm dried at 120 °C in air for 12 h. Galvanostatic charge–discharge tests were carried out in CR2016 type coin cells, and the cells were assembled in a glove-box filled with Ar atmosphere. For the half-cell test, metallic sodium was used as the anode. The electrolyte solution was 1 M NaClO4-ethylene carbonate (EC)–diethyl carbonate (DEC) (1
20. Subsequently, the slurry was cast uniformly on aluminum foil. The electrode film was vacuum-dried at 80 °C for 10 h to remove the solvent before roll-pressing. The electrode film was then punched into discs with diameters of 12 mm dried at 120 °C in air for 12 h. Galvanostatic charge–discharge tests were carried out in CR2016 type coin cells, and the cells were assembled in a glove-box filled with Ar atmosphere. For the half-cell test, metallic sodium was used as the anode. The electrolyte solution was 1 M NaClO4-ethylene carbonate (EC)–diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). The cut-off voltages were 1.2 V and 3.2 V. The galvanostatic electrochemical test was evaluated under an automatic battery testing system (LAND CT2001A model). The cyclic voltammetry (CV) tests were carried out on CH Instruments electrochemical workstation (CHI 660D) at the rate of 1 mV s−1 between 1.2 V and 3.2 V.
1 by volume). The cut-off voltages were 1.2 V and 3.2 V. The galvanostatic electrochemical test was evaluated under an automatic battery testing system (LAND CT2001A model). The cyclic voltammetry (CV) tests were carried out on CH Instruments electrochemical workstation (CHI 660D) at the rate of 1 mV s−1 between 1.2 V and 3.2 V.
3. Results and discussion
Fig. 1a gives the FT-IR spectra of as-prepared PNTCDA, where all the characteristic absorption bands of the imide group can be detected clearly. These bands at 1703 cm−1, 1670 cm−1 and 771 cm−1 were assigned to νas(imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), νs(imide C
O), νs(imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and δ(imide C
O), and δ(imide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The bands at 1350 cm−1 and 1582 cm−1 can be indexed to ν(imide C–N) and naphthalene, respectively. The result from FT-IR is consistent with previous reports, and thus demonstrates the successful synthesis of the target product (i.e. PNTCDA). Fig. 1b shows the cyclic voltammetry (CV) results of the PNTCDA. From the CV curves it is obvious that both the oxidation and reduction processes consist of two continuous steps, which are possibly associated with the formation of the radical anion (I−), the dianion (I2−),24 respectively, in a similar manner as that for it in Li-ion battery.21 The results of CV are quite consistent with the charge–discharge profiles in the Fig. 1c, in which there are two slope voltage curves of about 2.1 V and 2.4 V in the charge profile, and two slope voltage curves of about 1.8 V and 2.25 V in the discharge profile. As shown in Fig. 1c, at the low current density of 70 mA g−1, PNTCDA displays a reversible capacity of 150 mA h g−1 which is only approximately the half of the theoretical capacity of PNTCDA (279.2 mA h g−1 calculated according to the reaction shown in Scheme 1). Ideally, each formula unit will transfer two electrons in each step, and two steps are involved, so there should be four steps in CV curves. However, the experimental results of CV do not support this reaction mode given in Scheme 1. Obviously, the full electron transfer can not be achieved over the discharge–charge process. According to those previous studies about this material for Li-ion batteries,21 the full capacity may be obtained at a deeper discharge below 1.2 V, while which is accompanied by serious structural damage.
O). The bands at 1350 cm−1 and 1582 cm−1 can be indexed to ν(imide C–N) and naphthalene, respectively. The result from FT-IR is consistent with previous reports, and thus demonstrates the successful synthesis of the target product (i.e. PNTCDA). Fig. 1b shows the cyclic voltammetry (CV) results of the PNTCDA. From the CV curves it is obvious that both the oxidation and reduction processes consist of two continuous steps, which are possibly associated with the formation of the radical anion (I−), the dianion (I2−),24 respectively, in a similar manner as that for it in Li-ion battery.21 The results of CV are quite consistent with the charge–discharge profiles in the Fig. 1c, in which there are two slope voltage curves of about 2.1 V and 2.4 V in the charge profile, and two slope voltage curves of about 1.8 V and 2.25 V in the discharge profile. As shown in Fig. 1c, at the low current density of 70 mA g−1, PNTCDA displays a reversible capacity of 150 mA h g−1 which is only approximately the half of the theoretical capacity of PNTCDA (279.2 mA h g−1 calculated according to the reaction shown in Scheme 1). Ideally, each formula unit will transfer two electrons in each step, and two steps are involved, so there should be four steps in CV curves. However, the experimental results of CV do not support this reaction mode given in Scheme 1. Obviously, the full electron transfer can not be achieved over the discharge–charge process. According to those previous studies about this material for Li-ion batteries,21 the full capacity may be obtained at a deeper discharge below 1.2 V, while which is accompanied by serious structural damage.
Fig. 1d shows the charge–discharge curves of PNTCDA electrode at varying currents from 140 mA g−1 (1 C) to 2520 mA g−1 (30 C), and the capacities achieved at different currents were summarized in Fig. 1e. As shown in Fig. 1d and e, the PNTCDA electrode delivers a reversible capacity of 140 mA h g−1 at the rate of 1 C (140 mA h g−1). Even at high rate of 30 C (2520 mA g−1), which corresponds to a time of 2 min to fully discharge, the capacity was about 60% of that at the 1 C rate, implying that the present electrode material is suitable for high-power battery application. Obviously, the voltage gap between charge and discharge increases with the growth of current densities (Fig. 1d), which is owing to the internal resistance of the PNTCDA electrode. The cyclic performance of the PNTCDA electrode at the rate of 1 C (140 mA g−1) is given in Fig. 1f. It can be observed that the initial coulombic efficiency is as high as 97.6% which is much superior to that of those small molecule or monomer organic electrode materials. Furthermore, the PNTCDA electrode exhibits a very stable cyclic performance with almost indiscernible capacity decay and kept its coulombic efficiency around 100% during successive 500 cycles. It can be assumed that the excellent cyclic ability arises apparently from the electrochemical reversibility of the conjugated carbonyl group and the structural stability of the polyimide chains.
Encouraged by the suitable redox activities of PNTCDA polymer, we used this anode material to construct two full Na-ion batteries with Na3V2(PO4)3/C cathode and Na4Fe(CN)6/C cathode, respectively, which have been invented as promising cathode materials for sodium battery.2,5 Fig. 2a shows the charge–discharge curves of the Na3V2(PO4)3/C-Na half-cell at a current rate of 1 C in the voltage range of 2.5–4.0 V, Fig. 2b displays the charge–discharge profiles of Na4Fe(CN)6/C-Na half-cell between 2.0 and 3.8 V at the current rate of 1 C. As shown in the Fig. 2, the experimental capacity of Na3V2(PO4)3/C and Na4Fe(CN)6/C were 85 mA h g−1 and 75 mA h g−1, hence the full cell can be assembled with a mole ratio of Na3V2(PO4)3/C (cathode) to PNTCDA (anode) 1.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and Na4Fe(CN)6/C (cathode) to PNTCDA (anode) 1.87
1, and Na4Fe(CN)6/C (cathode) to PNTCDA (anode) 1.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The electrolyte solution was 1.0 mol L−1 NaClO4-ethylene carbonate (EC)–diethyl carbonate (DEC) (1
1. The electrolyte solution was 1.0 mol L−1 NaClO4-ethylene carbonate (EC)–diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). Coin type PNTCDA-Na3V2(PO4)3/C and PNTCDA-Na4Fe(CN)6/C full cells are measured at the voltage interval of 0–2.3 V and the achieved results are summarized in Fig. 3. As shown in Fig. 3a and c the cell works very well with an average voltage of about 1.2 V and fully realizes the reversible capacity both the anode and cathode materials, the capacities were calculated based on the anode materials. If based on the sum of anode and cathode materials, the capacity should be about 75 mA h g−1 and 70 mA h g−1 of PNTCDA-Na3V2(PO4)3/C and PNTCDA-Na4Fe(CN)6/C full cells respectively. In addition, these two cells also exhibit a good cycling stability with 75% and 65% capacity retention over 100 cycles at 1 C rate (Fig. 3b and d). While in the full aqueous sodium ion battery with PNTCDA as anode material, its cycle stability is not so good for the poor cycle performance of cathode material.22
1 by volume). Coin type PNTCDA-Na3V2(PO4)3/C and PNTCDA-Na4Fe(CN)6/C full cells are measured at the voltage interval of 0–2.3 V and the achieved results are summarized in Fig. 3. As shown in Fig. 3a and c the cell works very well with an average voltage of about 1.2 V and fully realizes the reversible capacity both the anode and cathode materials, the capacities were calculated based on the anode materials. If based on the sum of anode and cathode materials, the capacity should be about 75 mA h g−1 and 70 mA h g−1 of PNTCDA-Na3V2(PO4)3/C and PNTCDA-Na4Fe(CN)6/C full cells respectively. In addition, these two cells also exhibit a good cycling stability with 75% and 65% capacity retention over 100 cycles at 1 C rate (Fig. 3b and d). While in the full aqueous sodium ion battery with PNTCDA as anode material, its cycle stability is not so good for the poor cycle performance of cathode material.22
 | ||
| Fig. 2 (a) Charge–discharge curves of Na3V2(PO4)3/C-Na half-cell at 85 mA g−1 (1 C). (b) Charge–discharge curves of Na4Fe(CN)6/C-Na half-cell at 75 mA g−1 (1 C). | ||
4. Conclusion
In summary, we propose the use of PNTCDA, a kind of polyimide, as anode material for rechargeable sodium batteries. The two-electron transfer of each monomer unit results in a specific discharge capacity of around 140 mA h g−1, a discharge voltage of 1.7–2.4 V, and a superior high rate capability with 60% capacity delivery at a 32 C rate. Moreover, the intrinsic stability and insolubility of the polyimide ensures that it is not dissolved in the electrolyte, and thus allows an excellent cycling stability and a high initial coulomb efficiency of 97.6%. And we have successfully constructed two 1.2 V full Na-ion batteries by use of PNTCDA anode, which present a good cyclability and reversibility. Particularly, the environmental friendliness and low cost of this anodic material enable it to be used for large-scale electric storage applications.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (no. 21333002 and 20925312), and Shanghai Science & Technology Committee (13JC1407900).References
- M. Armand and J. M. Tarascon, Building better batteries, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- Z. Jian, L. Zhao, H. Pan, Y. S. Hu, H. Li, W. Chen and L. Chen, Electrochem. Commun., 2012, 14, 86–89 CrossRef CAS PubMed.
- B. L. Ellis, W. R. M. Makahnouk, Y. Makimura, K. Toghill and L. F. Nazar, Nat. Mater., 2007, 6, 749–753 CrossRef CAS PubMed.
- Y. L. Cao, L. F. Xiao, W. Wang, D. W. Choi, Z. M. Nie, J. G. Yu, L. V. Saraf, Z. G. Yang and J. Liu, Adv. Mater., 2011, 23, 3155–3160 CrossRef CAS PubMed.
- J. Qian, M. Zhou, Y. Cao, X. Ai and H. X. Yang, Adv. Energy Mater., 2012, 2, 410–414 CrossRef CAS.
- S. Komaba, T. Nakayama, A. Ogata, T. Shimizu, C. Takei, S. Takada, A. Hokura and I. Nakai, ECS Trans., 2009, 16, 43–55 CAS.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859–3867 CrossRef CAS.
- K. Tang, L. Fu, R. J. White, L. Yu, M. M. Titirici, M. Antonietti and J. Maier, Adv. Energy Mater., 2012, 2, 873–877 CrossRef CAS.
- J. F. Qian, Y. Chen, L. Wu, Y. L. Cao, X. P. Ai and H. X. Yang, Chem. Commun., 2012, 48, 7070–7072 RSC.
- J. F. Qian, X. Y. Wu, Y. L. Cao, X. P. Ai and H. X. Yang, Angew. Chem., Int. Ed., 2013, 52, 1–5 CrossRef.
- L. Zhao, H. L. Pan, Y. S. Hu, H. Li and L. Chen, Chin. Phys. B, 2012, 21, 028201 CrossRef.
- M. Armand, S. Grugeon, H. Vezin, S. Laruelle, P. Ribière, P. Poizot and J. M. Tarascon, Nat. Mater., 2009, 8, 120–125 CrossRef CAS PubMed.
- M. Zhou, J. F. Qian, X. P. Ai and H. X. Yang, Adv. Mater., 2011, 23, 4913–4917 CrossRef CAS PubMed.
- T. Suga, S. Sugita, H. Ohshiro, K. Oyaizu and H. Nishide, Adv. Mater., 2011, 23, 751–754 CrossRef CAS PubMed.
- L. Zhao, J. Zhao, Y. S. Hu, H. Li, Z. B. Zhou, M. Armand and L. Q. Chen, Adv. Energy Mater., 2012, 2, 962–965 CrossRef CAS.
- A. Abouimrane, W. Weng, H. Eltayeb, Y. Cui, J. Niklas, O. Poluektov and K. Amine, Energy Environ. Sci., 2012, 5, 9632–9638 CAS.
- R. R. Zhao, Y. L. Cao, X. P. Ai and H. X. Yang, J. Electroanal. Chem., 2013, 688, 93–97 CrossRef CAS PubMed.
- Y. Park, D. S. Shin, S. H. Woo, N. S. Choi, K. H. Shin, S. M. Oh, K. T. Lee and S. Y. Hong, Adv. Mater., 2012, 24, 3562–3567 CrossRef CAS PubMed.
- Z. P. Song, H. Zhan and Y. H. Zhou, Chem. Commun., 2009, 448–450 RSC.
- W. W. Deng, X. M. Liang, X. Y. Wu, J. F. Qian, Y. L. Cao, X. P. Ai, J. W. Feng and H. X. Yang, Sci. Rep., 2013, 3, 2671–2677 Search PubMed.
- Z. P. Song, H. Zhan and Y. H. Zhou, Angew. Chem., Int. Ed., 2010, 49, 8444–8448 CrossRef CAS PubMed.
- H. Qin, Z. P. Song, H. Zhan and Y. H. Zhou, J. Power Sources, 2014, 249, 367–372 CrossRef CAS PubMed.
- M. K. Ghosh and K. L. Mittal, Polyimides: fundamentals and applications, Marcel Dekker, New York, 1996 Search PubMed.
- C. De Luca, C. Giomini and L. Rampazzo, J. Electroanal. Chem., 1990, 280, 145–157 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |