Adding ethanol can effectively enhance the graphene concentration in water–surfactant solutions†
Abstract
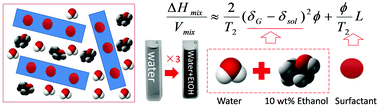
We demonstrate that in water–surfactant solutions, adding ethanol can lead to graphene dispersions of high concentration (∼0.46 mg mL−1). For both ionic and non-ionic surfactants, ∼10 wt% addition of ethanol is found to enhance the exfoliation efficiency and thus the graphene concentration up to three times. This enhancement is attributed to the combination of decreasing mixing enthalpy by ethanol addition and enhancing stability of surfactants. This method opens a whole new vista for preparing high-concentration graphene dispersions.

 Please wait while we load your content...
Please wait while we load your content...