Gallium(iii) xanthate as a novel thermal latent curing agent for an epoxy resin composite†
Abstract
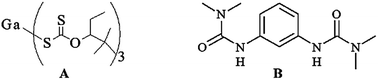
The latent thermal curing catalyst “Ga(III) xanthate” for an epoxy resin/phenol composite, and its curing behaviour are reported. Ga(III) xanthate swiftly cures the epoxy composite within 38.2 s at 200 °C. However the curing time of the epoxy composite with gallium(III) xanthate did not change after storage for six months unlike the commercially used catalyst.

 Please wait while we load your content...
Please wait while we load your content...