Graphene oxide-based electrochemical sensor: a platform for ultrasensitive detection of heavy metal ions†
Xuezhong Gonga,
Yunlong Bia,
Yihua Zhaoa,
Guozhen Liub and
Wey Yang Teoh*a
aClean Energy and Nanotechnology (CLEAN) Laboratory, School of Energy and Environment, City University of Hong Kong, Kowloon, Hong Kong S.A.R.. E-mail: wyteoh@cityu.edu.hk; Fax: +852-3442 0688; Tel: +852-3442 4627
bKey Laboratory of Pesticide and Chemical Biology of Ministry of Education, College of Chemistry, Central China Normal University, Wuhan, P. R. China
First published on 14th May 2014
Abstract
Facile functionalization of graphene oxide sheets on gold surface results in complexation-enhanced electrochemical detection of heavy metal ions, shown here for Pb2+, Cu2+ and Hg2+, with improved detection limits by two orders of magnitude relative to the control electrode.
The applications of graphene in nanotechnology-based devices have received unceasing interests since its discovery, attributed to its unique and often outstanding physicochemical and electronic properties.1 For electronic devices, extremely high quality graphene with little defects and oxygenated groups is necessary, and this is commonly synthesized through very delicate chemical vapor synthesis often with low yield.2 As to chemical applications, the trends in recent years have been focusing on the adaptation of reduced-graphene oxide (rGO), a lower quality variant of the graphene analogue, which can be obtained from the chemical reduction of oxidized and exfoliated graphite.3 For an even simpler and cheaper processing, graphene oxide (GO) sheets, which are obtained right after the oxidation/exfoliation of graphite without a further reduction step, can be explored for various applications such as hybrid functional materials,4 drug-delivery vehicles,5 and fluorescence-based sensors.6 This is despite the much lower electrical conductivity (at least two order of magnitudes) of GO compared to rGO.7 Nevertheless, given its large specific surface area and strong hydrophilic nature, GO shows great potential in the removal of aqueous pollutants,8 especially for a wide range of heavy metal ions.9 For example, the strong interactions of Cu2+ with GO surface make it an excellent adsorbent material,10 while at the same time enhancing the electronic conductivity through the binding of metal ions to the oxygen moieties on GO surface.11 In the same way, Ca2+ and Mg2+ can be deliberately added as cross-linkers to enhance the mechanical strength of graphene oxide composites.12
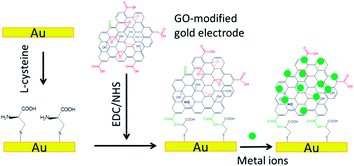
Following this train of thoughts, we design a GO-based electrochemical sensor on the basis of two important characteristics, that is, high adsorption of analytes and good conductivity thereafter. The GO was synthesized from the oxidation and exfoliation of purified graphite powders, following the well-established Hummers' method13 (see ESI†). The washed, filtered and dried brownish GO consists of densely mixed oxygenated groups, such as hydroxyl and epoxide (mostly on the basal surface), and carboxyl (mostly at the sheet edges).14 These oxygenated moieties provide avenues for direct modification by surface covalent functionalization.4,5,15 Using the ethyl(dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) mediated coupling strategy, the edges of GO, that is through the carboxyl groups, are attached directly onto a L-cysteine modified gold electrode surface (Scheme 1, see ESI† for detailed procedures). To the best of our knowledge, this is the first report of fabrication of a GO-based sensor using the facile EDC/NHS coupling strategy for the ultrasensitive detection of heavy metal ions. The attached GO sheets (see electron microscopy images, Fig. S2†) form the extended heterogeneous sites for the adsorption of metal ions, predominantly through the oxygenated sites as discussed below.
Wide scan X-ray photoelectron spectroscopy (XPS) confirms the presence of Au, S, C, N and O upon self-assembled monolayer attachment of L-cysteine on the bare gold surface (Fig. 1a). The carboxyl (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) and amide (–NH2) moieties are clearly evident from the C 1s binding energy peaks at 288.8 eV (Fig. 1b) and N 1s peak at 399.6 eV (Fig. 1c), under the respective narrow scans. Although not shown, the thiol group is also present, as measured from the S 2p binding energy peak at 162.0 eV. Subsequent attachment of GO resulted in the increased C and O intensity seen from the full scan in Fig. 1d, arising predominantly from the GO sheets. In the C 1s narrow scan (Fig. 1e), the peaks at 284.6, 286.0 and 288.5 eV were ascribed to the C
C–O) and amide (–NH2) moieties are clearly evident from the C 1s binding energy peaks at 288.8 eV (Fig. 1b) and N 1s peak at 399.6 eV (Fig. 1c), under the respective narrow scans. Although not shown, the thiol group is also present, as measured from the S 2p binding energy peak at 162.0 eV. Subsequent attachment of GO resulted in the increased C and O intensity seen from the full scan in Fig. 1d, arising predominantly from the GO sheets. In the C 1s narrow scan (Fig. 1e), the peaks at 284.6, 286.0 and 288.5 eV were ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O and C
C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of graphene oxide, respectively.16 Importantly, the peak at 287.6 eV which belongs to the O
O of graphene oxide, respectively.16 Importantly, the peak at 287.6 eV which belongs to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N shows the successful coupling of activated GO by EDC/NHS with cysteine.17 The weak binding energy peak at 284.8 eV was assigned to the C–S bond of the cysteine self-assembled monolayer on gold surface.18 The corresponding N 1s narrow scan is shown in Fig. 1f, where the amide nitrogen from the coupling reaction of –COOH and –NH2 is evident at 400.2 eV.19 The highest binding energy peak in the N 1s spectrum at 402.5 eV can be assigned to the nitrogen of unreacted NHS esters on the surface.20 The attachment of GO on gold surface was further confirmed electrochemically via the cyclic voltammogram of ferricyanide (Fig. S3†). The electron transfer resistance increases from 950 Ω for the L-cysteine modified gold electrode to 1360 Ω for the modified electrode after GO attachment (Fig. S4†).
C–N shows the successful coupling of activated GO by EDC/NHS with cysteine.17 The weak binding energy peak at 284.8 eV was assigned to the C–S bond of the cysteine self-assembled monolayer on gold surface.18 The corresponding N 1s narrow scan is shown in Fig. 1f, where the amide nitrogen from the coupling reaction of –COOH and –NH2 is evident at 400.2 eV.19 The highest binding energy peak in the N 1s spectrum at 402.5 eV can be assigned to the nitrogen of unreacted NHS esters on the surface.20 The attachment of GO on gold surface was further confirmed electrochemically via the cyclic voltammogram of ferricyanide (Fig. S3†). The electron transfer resistance increases from 950 Ω for the L-cysteine modified gold electrode to 1360 Ω for the modified electrode after GO attachment (Fig. S4†).
Cyclic voltammetry provides the initial qualitative analysis of as-prepared GO-modified gold electrode before and after adsorption of aqueous metal ions, hereby using Pb2+, Cu2+ and Hg2+ as the model analytes (Fig. S5†). We confirm that no redox peaks of the ions could be measured in the NH4Ac buffer electrolyte (pH 7.0) containing 50 mM KCl prior to the accumulation of heavy metal ions. After accumulation in an aliquot containing aqueous metal ions for 10 min (see Fig. S6†) followed by rinsing with metal-free NH4Ac buffer, the redox peaks for Pb2+, Cu2+ and Hg2+ appeared at the expected positions of −0.11 V/−0.23 V vs. Ag/AgCl,21 0.25 V/0.17 V vs. Ag/AgCl,19,21b and 0.55 V/0.51 V vs. Ag/AgCl,22 respectively (Fig. S5†).
For quantitative analysis, the square-wave voltammetry (SWV), which has a higher sensitivity compared to the CV technique, is employed under the same electrolyte condition as mentioned above. It is well known that the increased sensitivity of SWV arises from the larger net current (the difference between forward current and reverse current) with very little non-Faradaic and charging currents.23 Fig. 2 shows the calibration curve, measured on the anodic sweep, for (a) Pb2+, (b) Cu2+ and (c) Hg2+, over the GO-modified sensor. Here, regions of linear detection range are evident for all three metal ions, with detection limits as low as sub-ppb levels for the GO-modified electrode, that is, 0.4 ppb for Pb2+, 0.8 ppb for Hg2+ and 1.2 ppb for Cu2+. These minimum limits are much lower than the guideline values (10 ppb Pb2+, 6 ppb Hg2+ and 2000 ppb Cu2+) for drinking water given by the World Health Organization (WHO),24 and in fact, two orders of magnitude lower than that of the L-cysteine/gold electrode control, that is, 20 ppb for Pb2+, 10 ppb for Hg2+ and 50 ppb for Cu2+ (Table 1). The ultrahigh sensitivity is attributed to the accumulation of metal ions on the GO surface (shown below), giving rise to an improved Faradaic to capacitive current ratio (signal-to-noise, S/N ratio).25 It is interesting to note that the maximum limits of the linear range, 12.8 ppb for Hg2+, 51.2 ppb for Pb2+ and 200 ppb Cu2+, are inversely proportional to the adsorption capacity of GO, suggesting electronic interactions between the adsorbed metal ions when brought to close proximity on GO.26
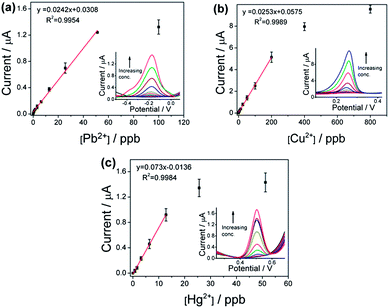 | ||
| Fig. 2 Calibration plots of SWV peak current of (a) Pb2+, (b) Cu2+ and (c) Hg2+ over GO-modified gold electrode. Insets show the corresponding SWV sweep. See Fig. S8† for reproducibility measurements for both minimum and maximum linear range detection limits. | ||
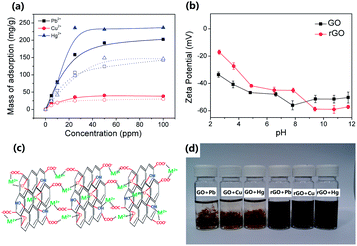
The Langmuir adsorption isotherms of Pb2+, Cu2+ and Hg2+ on GO are shown in Fig. 3a. The saturation adsorption capacity was estimated at 204, 270, and 41 mg metal ions per g GO for Pb2+, Hg2+ and Cu2+, respectively, which is much higher than other carbon analogues including reduced graphene oxide.27 Previous reports focused either on electrostatic attraction26 or metal coordination10 between GO and the analyte ions as the possible mode of adsorption. The former appears straightforward given the highly negative zeta potential of GO (Fig. 3b) that would readily attract the positive divalent analyte metal ions. However, a comparison with rGO, which is also characterized by similar zeta potential profile but with reduced oxygenated moieties, shows a lower adsorption capacity of 149, 178, and 31 mg metal ions per g rGO for Pb2+, Hg2+ and Cu2+, respectively. Quantification of O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio by XPS revealed an oxygen content three times higher for GO (O
C ratio by XPS revealed an oxygen content three times higher for GO (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : C = 2.7) compared to rGO (O
: C = 2.7) compared to rGO (O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : C = 0.09). Note: purification of graphite source prior to Hummers' treatment is necessary to achieve a high O
: C = 0.09). Note: purification of graphite source prior to Hummers' treatment is necessary to achieve a high O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio. However, the adsorption capacities of metal ions on rGO easily exceed above 70% of that on GO. In other words, we believe a mixed electrostatic attraction and metal coordination (Fig. 3c) may have taken place, the latter especially dominant on GO. This is further evidenced by the significant aggregation of GO upon addition of metal ions as a result of metal coordination and cross-linking of GO sheets (Fig. 3d). By comparison, rGO is only slightly aggregated and the suspension remains stable.
C ratio. However, the adsorption capacities of metal ions on rGO easily exceed above 70% of that on GO. In other words, we believe a mixed electrostatic attraction and metal coordination (Fig. 3c) may have taken place, the latter especially dominant on GO. This is further evidenced by the significant aggregation of GO upon addition of metal ions as a result of metal coordination and cross-linking of GO sheets (Fig. 3d). By comparison, rGO is only slightly aggregated and the suspension remains stable.
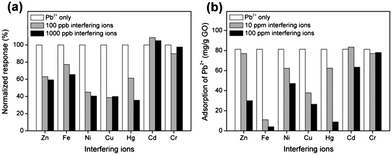
Using Pb2+ as the reference analyte, we further assess the selectivity of the GO-modified electrode. In principle, selective detection requires the covalent attachment of ion-specific antibodies, proteins or polymers28 through the functionalization of the oxygen moieties, which is beyond the scope of the present study. Instead, it is interesting to investigate the selectivity of the “bare” GO as is, which provides further insights on its sensing characteristics. Fig. 4a shows the effects of the addition of various individual interfering ions, that is, Zn2+, Ni2+, Fe3+, Cd2+, Cr6+, Cu2+ and Hg2+, during the detection of 25 ppb Pb2+. Each test was carried out individually with one interfering ion at low (100 ppb) and high (1000 ppb) concentrations (Fig. S9†). We found that the addition of Zn2+, Ni2+, Fe3+, Cu2+ and Hg2+ significantly reduced the sensing response of Pb2+ at both low and high concentrations of the interfering ions. Essentially, these interfering divalent cations compete with Pb2+ ions for adsorption on GO through electrostatic attraction and coordination on the oxygenated sites. Along the same analogy, minimal interference was observed in the case of Cr6+ in the form of chromate (Cr2O72−), where there is a lack of Cr valance d-orbital for the coordination with the lone pair electrons of oxygen on GO. In fact, the electrostatic repulsion between Cr2O72− and the negatively charged GO kept the anions separated. Cd2+ has little influence (in fact slightly positive) on the sensing response of Pb2+ due to the weak coordination and similar redox potential as discussed below.29
As competitive adsorption has been identified, Fig. 4b shows the net adsorption of 10 ppm Pb2+ on GO in the presence of the abovementioned interfering ions. Since direct quantification of adsorption of Pb2+ on the GO-modified electrode at an ultralow sensing concentration may not be so straightforward, the studies of adsorption interference were conducted in relevance to the Langmuir isotherm. At the reference concentration of 10 ppm, adsorbed Pb2+ exists as a submonolayer on GO (Fig. 3a), which in principle allows for sufficient buffer adsorption sites in the presence of low concentration of foreign ions. As shown in Fig. 4b, the degree of Pb2+ adsorption interference is varied for the different detrimental ions Zn2+, Fe3+, Ni2+, Cu2+ and Hg2+. Despite being in the submonolayer range, the presence of a low concentration (10 ppm) of Fe3+ significantly reduced the adsorption of Pb2+. This is prompted by the stronger Coulombic force of attraction between Fe3+ and the oxygenated moieties of GO (2.1 times higher compared to Pb2+). On the contrary, Zn2+ exhibits little adsorption interference at low concentration but at high concentration (100 ppm), Pb2+ adsorption is retarded by more than 60%, implying competitive adsorption when the Langmuir sites become limiting. The same applies to Hg2+. Based on the different extent of adsorption interference, it appears that sensing response is not necessarily proportional to the net adsorbed Pb2+. For example, a much lower sensing response of Pb2+ in the presence of Fe3+ would be expected relative to Zn2+, but this is clearly not the case (Fig. 4a). Likewise, the stronger adsorption interference of Cu2+ relative to Ni2+ was not reflected in the sensing response. The results imply adsorption-induced galvanic interference from the secondary ions during the sensing of Pb2+ on GO. Galvanic oxidation of Pb by highly electronegative analytes, i.e., Cu2+ and Hg2+, resulted in a reduced Pb2+ stripping signal, more so than in the presence of electropositive interfering ions such as Zn2+, Fe3+ and Ni2+. Since the electrostatic repulsion of Cr2O72− kept Cr6+ away from the GO surface, it did not interfere with the adsorption and sensing response of Pb2+. Cd2+ shows negligible adsorption interference at low concentration due to the soft acid nature of Cd2+ relative to Pb2+, and only became obvious at high concentration. Because of the relatively weak interaction, it shows minimal interference to Pb2+ sensing.
Finally, the reproducibility of GO-modified electrodes was assessed by comparing the stripping peak current of 50 ppb Pb2+, 25 ppb Cu2+ and 10 ppb Hg2+ using electrodes fabricated on different occasions (n = 5). In all cases, excellent reproducibility of sensing response was achieved, with relative standard deviations (RSDs) of 2.1% for Pb2+, 3.8% for Cu2+ and 3.3% for Hg2+ respectively, revealing the high reproducibility of the electrodes (Fig. S10†).
Conclusions
In summary, we have showcased the facile fabrication of a highly reproducible GO-based electrochemical sensor for the ultrasensitive detection of heavy metal ions. The high sensitivity arises from the strong adsorption of these heavy metal ions on GO, both electrostatically and through metal coordination on oxygenated sites. In this respect, it is more beneficial than rGO, which lacks the oxygenated group active sites compared to GO (and is also simpler to process compared to rGO). In general, the showcase device provides a universal platform for which further functionalization with analyte-specific proteins, antibodies and polymers can be achieved through covalent attachment on the oxygenated moieties for high performance sensors.Acknowledgements
The authors acknowledge the financial support of Hong Kong UGC through the General Research Fund (CityU 104812) and City University of Hong Kong through Startup Grant (7200252).Notes and references
- (a) A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed; (b) J. Wu, W. Pisula and K. Mullen, Chem. Rev., 2007, 107, 718–747 CrossRef CAS PubMed.
- (a) X. S. Li, W. W. Cai, J. H. An, S. Y. Kim, J. H. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed; (b) D. H. Geng, B. Wu, Y. L. Guo, L. P. Huang, Y. Z. Xue, J. Y. Chen, G. Yu, L. Jiang, W. P. Hu and Y. Q. Liu, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 7992–7996 CrossRef CAS PubMed.
- (a) S. Park, J. An, I. Jung, R. D. Piner, S. J. An, X. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed; (b) S. Some, Y. Kim, Y. Yoon, H. Yoo, S. Lee, Y. Park and H. Lee, Sci. Rep., 2013, 3, 1–3 Search PubMed.
- S. Park, D. A. Dikin, S. T. Nguyen and R. S. Ruoff, J. Phys. Chem. C, 2009, 113, 15801–15804 CAS.
- Z. Liu, J. T. Robinson, X. M. Sun and H. J. Dai, J. Am. Chem. Soc., 2008, 130, 10876–10877 CrossRef CAS PubMed.
- C. H. Liu, Z. Wang, H. X. Jia and Z. P. Li, Chem. Commun., 2011, 47, 4661–4663 RSC.
- W. Gao, L. B. Alemany, L. J. Ci and P. M. Ajayan, Nat. Chem., 2009, 1, 403–408 CrossRef CAS PubMed.
- (a) S. T. Yang, S. Chen, Y. Chang, A. Cao, Y. Liu and H. Wang, J. Colloid Interface Sci., 2011, 359, 24–29 CrossRef CAS PubMed; (b) P. Bradder, S. K. Ling, S. B. Wang and S. M. Liu, J. Chem. Eng. Data, 2011, 56, 138–141 CrossRef CAS.
- (a) C. Xu, X. Wang, L. Yang and Y. Wu, J. Solid State Chem., 2009, 182, 2486–2490 CrossRef CAS PubMed; (b) M. Machida, T. Mochimaru and H. Tatsumoto, Carbon, 2006, 44, 2681–2688 CrossRef CAS PubMed.
- (a) S. T. Yang, Y. Chang, H. Wang, G. Liu, S. Chen, Y. Wang, Y. Liu and A. Cao, J. Colloid Interface Sci., 2010, 351, 122–127 CrossRef CAS PubMed; (b) C. Xu, X. Wang, L. C. Yang and Y. P. Wu, J. Solid State Chem., 2009, 182, 2486–2490 CrossRef CAS PubMed.
- M. Mathesh, J. Q. Liu, N. D. Nam, S. K. H. Lam, R. K. Zheng, C. J. Barrow and W. R. Yang, J. Mater. Chem. C, 2013, 1, 3084–3090 RSC.
- S. J. Park, K. S. Lee, G. Bozoklu, W. W. Cai, S. T. Nguyen and R. S. Ruoff, ACS Nano, 2008, 2, 572–578 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- (a) H. Yang, S. V. Kershaw, Y. Wang, X. Gong, S. Kalytchuk, A. L. Rogach and W. Y. Teoh, J. Phys. Chem. C, 2013, 117, 20406–20414 CrossRef CAS; (b) D. R. Dreyer, S. J. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC; (c) H. He, J. Klinowski, M. Forster and A. Lerf, Chem. Phys. Lett., 1998, 287, 53–56 CrossRef CAS.
- (a) L. M. Veca, F. Lu, M. J. Meziani, L. Cao, P. Zhang, G. Qi, L. Qu, M. Shrestha and Y. P. Sun, Chem. Commun., 2009, 2565–2567 RSC; (b) S. Wang, P. J. Chia, L. L. Chua, L. H. Zhao, R. Q. Png, S. Sivaramakrishnan, M. Zhou, R. G. S. Goh, R. H. Friend, A. T. S. Wee and P. K.-H. Ho, Adv. Mater., 2008, 20, 3440–3446 CrossRef CAS; (c) H. Yang, F. Li, C. Shan, D. Han, Q. Zhang, L. Niu and A. Ivaska, J. Mater. Chem., 2009, 19, 4632–4638 RSC.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS PubMed.
- E. Chow, E. L. S. Wong, T. Bocking, Q. T. Nguyen, D. B. Hibbert and J. J. Gooding, Sens. Actuators, B, 2005, 111, 540–548 CrossRef PubMed.
- G. Dodero, L. De Michieli, O. Cavalleri, R. Rolandi, L. Oliveri, A. Dacca and R. Parodi, Colloids Surf., A, 2000, 175, 121–128 CrossRef CAS.
- G. Liu, Q. T. Nguyen, E. Chow, T. Böcking, D. B. Hibbert and J. J. Gooding, Electroanalysis, 2006, 18, 1141–1151 CrossRef CAS.
- (a) T. Bocking, M. James, H. G. L. Coster, T. C. Chilcott and K. D. Barrow, Langmuir, 2004, 20, 9227–9235 CrossRef PubMed; (b) E. Delamarche, G. Sundarababu, H. Biebuyck, B. Michel, C. Gerber, H. Sigrist, H. Wolf, H. Ringsdorf, N. Xanthopoulos and H. J. Mathieu, Langmuir, 1996, 12, 1997–2006 CrossRef CAS.
- (a) E. Chow, D. B. Hibbert and J. J. Gooding, Anal. Chim. Acta, 2005, 543, 167–176 CrossRef CAS PubMed; (b) N. Kong, J. Q. Liu, Q. S. Kong, R. Wang, C. J. Barrow and W. R. Yang, Sens. Actuators, B, 2013, 178, 426–433 CrossRef CAS PubMed.
- (a) Z. Q. Zhu, Y. Y. Su, J. Li, D. Li, J. Zhang, S. P. Song, Y. Zhao, G. X. Li and C. H. Fan, Anal. Chem., 2009, 81, 7660–7666 CrossRef CAS PubMed; (b) H. Zhou, X. Wang, P. Yu, X. M. Chena and L. Q. Mao, Analyst, 2012, 137, 305–308 RSC.
- J. Osteryoung and J. J. O'Dea, Electroanalytical Chemistry, Marcel Dekker, New York, 1986, vol. 14 Search PubMed.
- World Health Organization (WHO), Guidelines for drinking-water quality, Sixty-first meeting, Rome, June 10–19 2003, ftp://ftp.fao.org/es/esn/jecfa/jecfa61sc.pdf Search PubMed.
- (a) J. Cassidy, J. Ghoroghchian, F. Sarfarazi, J. J. Smith and S. Pons, Electrochim. Acta, 1986, 31, 629 CrossRef CAS; (b) K. R. Wehmeyer, M. R. Deakin and R. M. Wightman, Anal. Chem., 1985, 57, 1913–1916 CrossRef CAS.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- (a) M. Imamoglu and O. Tekir, Desalination, 2008, 228, 108–113 CrossRef CAS PubMed; (b) Y. H. Li, S. Wang, J. Wei, X. Zhang, C. Xu and Z. Luan, Chem. Phys. Lett., 2002, 357, 263–266 CrossRef CAS; (c) Y. H. Li, J. Ding, Z. Luan, Z. Di, Y. Zhu and C. Xu, Carbon, 2003, 41, 2787–2792 CrossRef CAS; (d) Z. H. Huang, X. Y. Zheng, W. Lv, M. Wang, Q. H. Yang and F. Y. Kang, Langmuir, 2011, 27, 7558–7562 CrossRef CAS PubMed.
- (a) D. A. Blake, R. M. Jonesa, R. C. Blake, A. R. Pavlova, I. A. Darwish and H. N. Yu, Biosens. Bioelectron., 2001, 16, 799–809 CrossRef CAS; (b) Z. Q. Zhao, X. Chen, Q. Yang, J. H. Liu and X. J. Huang, Chem. Commun., 2012, 48, 2180–2182 RSC.
- E. Chow, D. Ebrahimi, J. J. Gooding and D. B. Hibbert, Analyst, 2006, 131, 1051–1057 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details on the preparation of GO-modified electrodes, physicochemical characterization and electrochemical measurements, CV and SWV of ferricyanide and heavy metal ions, calibration plots of heavy metal ions, SWV of Pb2+ in the presence of interfering ions, and reproducibility measurements of the GO-modified electrodes. See DOI: 10.1039/c4ra02247e |
| This journal is © The Royal Society of Chemistry 2014 |