Preparation of graphene oxide nano-composite ion-exchange membranes for desalination application†
Swati Gahlota,
Prem P. Sharmaa,
Hariom Guptaa,
Vaibhav Kulshrestha*ab and
Prafulla K. Jhac
aCSIR-Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Council of Scientific & Industrial Research (CSIR), Gijubhai Badheka Marg, Bhavnagar-364 002, Gujarat, India. E-mail: vaibhavk@csmcri.org; vaihavphy@gmail.com; Fax: +91-0278-2566970
bAcademy of Scientific and Innovative Research, CSIR-Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Council of Scientific & Industrial Research (CSIR), Gijubhai Badheka Marg, Bhavnagar-364 002, Gujarat, India
cDepartment of Physics, The M S University of Baroda, Vadodara, Gujarat, India
First published on 7th April 2014
Abstract
Nano-composite ion-exchange membranes (IEMs) consisting of graphene oxide (GO) (0.5, 1, 2, 5 and 10%) (w/w) and sulfonated polyethersulfone (SPES) of thickness 180 μm are prepared with enhanced electrochemical properties. In particular, the transport properties of SPES are favourably manipulated by the incorporation of GO. Intermolecular interactions between the components in composite membranes are established by FTIR. Membranes are characterized by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) which showed the uniform distribution of GO sheets in SPES matrix. The maximum ionic conductivity has been found in 10% GO composite with higher methanol crossover resistance and selectivity. Water desalination performance of the nano-composite membranes have been evaluated by ionic flux, power consumption and current efficiency during salt removal. 10% GO nano-composite membrane shows 3.51 mol m−2 h−1 ionic flux, 4.3 kW h kg−1 power consumption and 97.4% current efficiency for salt removal. The values of ionic flux and current efficiency are 19% and 12% higher respectively while 20% lower power consumption is observed as compared to SPES membrane. The strong interfacial interactions due to the insertion of GO nanofillers into the SPES matrix improve the thermal and mechanical properties of the nanocomposite membranes. Nano-composite membrane shows the better performance and higher stability which may be used for the practical application such as DMFC and electrodialysis.
Introduction
Energy and fresh water are demands on priority of today's world. Researchers are constantly developing techniques to fulfill such requirements. Fuel cell technology is one of the best alternate of conventional energy generation methods. Polymer electrolyte membrane fuel cells (PEMFC) are devices with advantage of high power density and better efficiency.1–4 Electrodialysis is a process for removal of ions from brackish or sea water to produce fresh water including waste water treatment to recover some valuable elements in chemical industry.5–7 Both the processes employ ion exchange membranes (IEMs). IEMs for electrodialysis and fuel cell must exhibit high ionic conductivity, high permselectivity for counter ions, high chemical stability and low fuel permeability.8,9 Different types of membranes have been developed suitable for the above applications using several polymer/organic or inorganic materials. Such kinds of membranes own the better physiochemical properties and also incorporate the advantages of membrane forming attribute of polymers.10,11 Different materials that are being used for nanocomposite membranes are silica, carbon nanotubes, graphene oxide etc. Composites of polymers with carbon nanotubes (CNT) have been reported for water treatment application.12 Vertically aligned CNTs have also been utilized to measure desalination efficiency under pressure driven conditions.13 SPEEK/silica membrane has been reported for direct methanol fuel cell (DMFC) application and shows better performance.14 Thus incorporation of organic or inorganic materials into the polymer matrix not only enhanced the properties but also expand the application region. Graphene oxide (GO) is emerging as an efficient material whose composite with polymer can be utilized for many applications such as biosensors, super capacitors, fuel cells etc.15–17 The fascinating properties of GO e.g. high surface area, excellent mechanical strength etc. makes it a distinct material to be used as organic filler for nanocomposite membrane.18,19 Poly(ethylene oxide) (PEO)/GO electrolyte membrane was prepared by Cao et al. for low temperature fuel cells.20 Polysulfone/GO membrane with enhanced hydrophilicity was prepared by B. M. Ganesh et al. and used for rejection of salts from water.21Herein, we demonstrate the fabrication of SPES/GO composite membrane for fuel cell and electrodialysis application. Membranes containing various weight content of GO (n = 0.5, 1, 2, 5 and 10%) are prepared and designated as SG-n. Physical and electrochemical characterizations of prepared membranes are made using different techniques. Investigations of ion exchange capacity (IEC), water uptake are done and their performance towards water desalination is presented in the manuscript.
Experimental
Materials and methods
Graphite powder is purchased from Sigma Aldrich. Poly(ether-sulfone), obtained from Solvay chemicals, India, is used after drying under vacuum. Other chemicals are obtained commercially and used as received without further purification.Graphene oxide is prepared using modified Hummer's method.17 Typically in a round bottom flask 1 g graphite, 1 g NaNO3 and 46 mL H2SO4 are taken and stirred for 2 h in an ice bath at 0–5 °C. 8 g KMNO4 was added in the above solution with stirring at room temperature for 4 h. Now 80 mL water is added drop wise with stirring for 3 h as it produces enormous amount of heat. Finally, 200 mL water and 5% H2O2 is added and washed repeatedly and centrifuged to recover GO. Sulfonation of PES was carried out as reported earlier using conc. H2SO4 (95–98%).22 Known amount of GO is dissolved in N,N′-dimethylacetamide and sonicated for 3–4 h for uniform dispersion. Definite quantity of SPES polymer is dissolved in the GO solution with stirring for 4 h followed by further sonication for 6 h. Resulting solution was cast on glass followed by vacuum drying. Membranes with different GO concentration (0, 0.5, 1, 2, 5 and 10%) (w/w) are prepared and designated as SPES, SG-05, SG-1, SG-2, SG-5 and SG-10 respectively. The schematic representation for graphite to composite membrane preparation is shown in Scheme 1. Polyethylene based styrene divinylbenzene anion exchange membrane is used for the study. The details for the membrane preparation are included in the ESI.†
Characterization of the membranes
The membranes are characterized by the means of chemical, structural, mechanical, thermal and electrochemical using different techniques. Details are included in the ESI.†Ionic and electronic conductivity of the membranes
Ionic conductivity measurements of the composite membranes are conducted on a potentiostat/galvanostat frequency response analyzer (Auto Lab, Model PGSTAT 30) in water at different temperatures ranging from 30 to 90 °C. Before measurements all the membranes are fully equilibrated with water. For the measurement the membranes are sandwiched between two circular stainless steel electrodes (1.0 cm2). The membrane resistances were obtained from Nyquist plots and the ionic conductivity (σ) was calculated from the equation:23where L is the distance between the electrodes used to measure the potential, R is the resistance of the membrane, and A is the surface area of the membrane.
The electronic conductivity of the dry membranes was measured with Keithley electrometer using the following equation:
Methanol crossover resistance
Resistance to methanol crossover for the membranes are evaluated by the measurement of the methanol permeability with two compartment cell. Details of the experiment and formula used are described in the ESI.†Desalination by electrodialysis
The performance of the prepared membranes is tested in a locally fabricated PVC based ED cell. The effective area of the membranes during ED was 60 cm2. The scheme of the ED setup and the membrane configuration in the cell are illustrated in Scheme 2, which consists of five compartments. Precious titanium oxide based electrodes was used as cathode and anode. A DC power source was used to apply a constant potential across the electrodes and the resulting current was recorded using a Aplab L1285 power supply. During ED 600 cm3 of 0.1 mol dm−3 NaCl feed solution was re-circulated at 3 L h−1 through the dilute (DC) and concentrated compartments (CC). The CC was adjacent to the electrode wash (EW) compartments in which 500 cm−3 of 0.1 mol dm−3 Na2SO4 was circulated at 3 L h−1. The ion concentration from each compartment was determined by a conductivity and pH measurements of the DC and CC during the ED process. The volume change in each reservoir was also recorded during the experiment. The performances of the prepared membranes were analyzed in terms of flux, current efficiency and energy consumption calculated by the following equations:6
 | (1) |
 | (2) |
 | (3) |
Results and discussion
Structural and chemical characterization
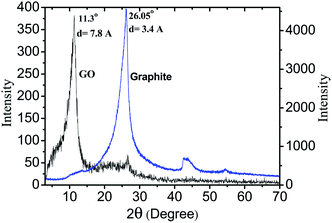
The graphite is converted into the GO by modified Hummer's methods and confirmed by the XRD and FTIR analysis. XRD spectra of GO showed the diffraction peak at 11.31° while the diffraction peak for graphite appeared at 26.05° well matched with the literature (Fig. 1).24,25 The interlayer spacing of GO increased by 7.81 Å to the initial value 3.4 Å for graphite. Also it can be seen from the figure that the peak shifts towards left and becomes broader which is due to the addition of functional groups at GO. The presence of a range of reactive groups such as carboxyl, hydroxyl, epoxy etc. on GO can be confirmed by FTIR analysis as shown in Fig. 2. FTIR spectra of prepared GO shows O–H stretching at 3402 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at 1717 cm−1, 1624 cm−1 and C–O stretching at 1051 cm−1.25 The presence of sulfonic acid groups in PES is confirmed by the FTIR analysis and the degree of sulfonation is found to be 60–65% as confirmed by 1H NMR.26 The interaction between polymer and GO is also determined by FTIR spectra. Fig. 2 illustrates the FTIR spectra of SPES and SG-10 membranes. The addition of functional groups is more in SG-10 as compared to the SPES. This is due to the presence of GO in SG-10 membrane which adds reactive groups onto the membrane matrix. XRD diffraction graphs of SPES and SG-10 membrane are shown in Fig. S-1.† The diffraction peak for SPES and SG-10 membrane are found to be at 22.21° and 17.96° respectively, the shift in peak is due to the presence of GO in SPES matrix.
O stretching at 1717 cm−1, 1624 cm−1 and C–O stretching at 1051 cm−1.25 The presence of sulfonic acid groups in PES is confirmed by the FTIR analysis and the degree of sulfonation is found to be 60–65% as confirmed by 1H NMR.26 The interaction between polymer and GO is also determined by FTIR spectra. Fig. 2 illustrates the FTIR spectra of SPES and SG-10 membranes. The addition of functional groups is more in SG-10 as compared to the SPES. This is due to the presence of GO in SG-10 membrane which adds reactive groups onto the membrane matrix. XRD diffraction graphs of SPES and SG-10 membrane are shown in Fig. S-1.† The diffraction peak for SPES and SG-10 membrane are found to be at 22.21° and 17.96° respectively, the shift in peak is due to the presence of GO in SPES matrix.
Fig. 3 shows the AFM, SEM and TEM images of GO. All three analyses show that GO have a flake-like structure. Fig. 4(A–C) shows the 3 dimensional AFM images of the prepared membranes. Surface roughness of each membrane was calculated using NT-MDT software which varies from 6.5 nm to 24.35 nm for SPES and SG-10 membrane respectively. Thus it reveals the increasing roughness value of the membranes with increasing the amount of GO. Fig. 4(D–G) shows the SEM images of SPES, SG-1, SG-5 and SG-10 membranes. Cross-sectional views of composite membranes demonstrate a layered structure of dispersed GO sheets into the SPES matrix. TEM images of GO composite membranes at different magnifications are shown in Fig. 4(H–J). The transparency of images shows the interaction between SPES and GO, and the uniform dispersion of GO into the SPES matrix (Fig. 5).
 | ||
| Fig. 3 AFM (A and B), SEM (C and D) and TEM (E and F) images of graphene oxide at different magnifications. | ||
 | ||
| Fig. 4 AFM images of (A) SPES (B) SG-1 (C) SG-10, SEM images of (D) SPES (E) SG-1 (F) SG-5 (G) SG-10 and TEM images (H)–(J) of graphene oxide/SPES composite at different magnifications. | ||
Thermal and mechanical characterization
Characterization of thermal properties of GO and nanocomposite membranes was done by using TGA. Fig. S-2† shows the TGA and DTG curve for the GO powder. Three step weight losses can be observed from the graph at 85, 215 and 293 °C (as obtained from DTG curve). Fig. S-3† demonstrates the TGA and DTG graphs of composite membranes respectively. As obtained by DTG graphs the maximum degradation temperature was found to be between 500–600 °C. Other two weight losses are observed in the range from 200–300 °C and 400–450 °C respectively. There is a gradual decrease in the thermal stability of membranes as the amount of GO increases which may be due to the presence of carboxyl and hydroxyl groups on GO's surface. The change from 200 to 300 °C is attributed to the degradation of functional groups present in the membrane.22 Thermal degradation from 500 to 600 °C can be described by decomposition of the backbone of the polymer chains. DSC graphs of GO composite membranes are shown in Fig. S-4† for all the prepared membranes. The melting peak of composite membranes shifts towards the higher temperature side by incorporating the GO amount into the SPES matrix. The thermo-mechanical studies of composite membranes are performed by DTMA analysis as shown in Fig. S-5.† Fig. S-5.† shows that the storage modulus and temperature of tan(δ) increases by increasing the GO content into the membrane.27,28 The GO flakes are working as the fillers for the SPES matrix and decreasing the pore size of the membrane, which provide the higher mechanical as well as thermal stability.Electrochemical properties
Water uptake (WU), dimensional stability (DS) and IEC are important parameters for ion exchange membranes. Table 1 show the WU, DC and IEC values of each membrane. The IEC of the composite membrane decreases slightly due to GO content into the membrane matrix and approached to 1.27 meq. g−1.18 The presence of carboxyl, hydroxyl group in GO maintains the IEC of the membranes. As can be seen from the table, WU increases from lower to higher GO concentration (from SPES to SG-10). SG-10 (10% GO) shows the maximum water uptake i.e. 15.19%. The important factor of the membrane is swelling, which decreases by incorporating the GO content in the SPES. This may be due to the reduction of pore size in the membrane as GO is acting as the filler. The bound water into the membrane is found to be increased by GO content, as calculated by TGA analysis of the membrane between 100–150 °C. SPES membranes show the lowest bound water content in comparison with all membranes while the SG-10 composite membrane shows the highest bound water, more bound water in membrane leads to a higher water retention ability.| Membrane | IEC (meq. g−1) | Water uptake % | Transport number (tm) | Bound water % | Free water % | Dimensional change % | Ionic cond. × 10−2 (S cm−1) | Electronic cond. × 10−2 (S cm−1) |
|---|---|---|---|---|---|---|---|---|
| SPES | 1.40 | 12.12 | 0.87 | 0.32 | 11.8 | 19.54 | 2.34 | 2.544 |
| SG-05 | 1.395 | 13.62 | 0.90 | 0.20 | 13.42 | 16.18 | 3.87 | 0.612 |
| SG-1 | 1.39 | 12.20 | 0.91 | 0.10 | 12.10 | 12.52 | 3.435 | 0.416 |
| SG-2 | 1.37 | 13.71 | 0.93 | 0.67 | 13.04 | 4.55 | 4.177 | 0.177 |
| SG-5 | 1.334 | 13.87 | 0.93 | 0.46 | 13.41 | 6.28 | 5.772 | 0.084 |
| SG-10 | 1.27 | 15.19 | 0.96 | 0.64 | 14.55 | 7.55 | 6.4 | 0.051 |
Ionic and electronic conductivity
In Table 1 the values of ion conductivities are shown for different membranes. The ionic conductivities of composite membranes are calculated by Nyquist plot. In the ionic conductivities of the GO/SPES membranes, with the GO particles introduced into the SPES matrix, the ionic conductivity of the GO/SPES membranes was increased regardless of the amounts of GO particles.29,20 The increment in the conductivity is due to the presence of carboxyl and hydroxyl groups present in GO particles. The effect of temperature on ionic conductivity is studied from 30 to 90 °C, on increasing the temperature the conductivity of the membranes also increases due to the higher ionic diffusion at higher temperature as shown in Fig. 6. Electronic conductivity of the composite membranes decreases as the GO content increases in the SPES matrix. SG-10 membrane has the lowest value, since addition of GO increases the insulating groups to the membranes because at higher concentration agglomeration occurs which reduces the electronic conductivity of the composite membranes as shown in Table 1.Methanol permeation (PM) resistance and selectivity of hybrid membranes
Low methanol permeation with high proton conductivity is the basic requirement for proton exchange membrane for DMFC application. Methanol permeability of SPES and GO/SPES membranes is shown in Fig. 7. It can be seen that the methanol permeability of the composite membrane decreased with the increment of the GO content. The GO is acting as the blocking agent for methanol and reducing the free volume of the composite membranes.18 The interaction between GO and SPES restricts the formation of the channels in membranes, which leads to low methanol permeability. The methanol permeability for the SPES membrane is found to be 1.827 × 10−7 cm2 S−1, which reduces to 1.44 × 10−7 cm2 S−1 for SG-1 and 1.412 × 10−7 cm2 S−1 for SG-5 membranes. Finally the methanol permeability value for SG-10 reached to 1.345 × 10−7 cm2 S−1. We also calculate the selectivity of the membrane. For the SPES it is found to be 1.724 × 105, which enhanced in the composite membranes and further reached to 6.321 × 105 for SG-10 membrane. The low methanol permeability and high selectivity of SG-10 membrane makes it applicable for fuel cells.Electrodialytic performance of composite ion exchange membranes
Current–voltage (I–V) characteristics for different composite membranes are studied in equilibration with 0.1 M NaCl solution and depicted in Fig. 8. The I–V curves show three typical characteristic regions, viz., ohmic, plateau and non-ohmic which reveal the information about the ion transport and concentration polarization phenomenon across the ion-exchange membranes.30,31 In the ohmic region the current density increases with applied potential due to the presence of a large number of ions, in the plateau region the current density remains constant with applied potential and in the non-ohmic region or over-limiting region ions get depleted causing water splitting and high current density. ΔV (plateau length), Δi, and Ilim values for composite membranes are calculated from Fig. 8 and presented in Table 2.31 For SPES/GO membranes the plateau length and Δi values increase with the increase in GO content. Relatively higher ΔV and Δi values for membranes with high fixed charge concentration indicate their high electro-transport of counter ions from the diffusive boundary layer to the membrane matrix and thus results in higher current for concentration polarization (Ilim). The transport of counter ions through an ion-exchange membrane leads to the difference in counter ion concentration on the two membrane surfaces, which in turn creates concentration polarization.32 The SG-10 membrane shows superior electro-transport properties and can be used for the water desalination.| Membrane | ΔV (V) | Δi (mA cm−2) | Ilim (mA cm−2) |
|---|---|---|---|
| SPES | 3.10 | 2.67 | 5.20 |
| SG-1 | 3.11 | 3.11 | 5.85 |
| SG-2 | 3.34 | 4.72 | 6.20 |
| SG-5 | 3.61 | 4.84 | 6.30 |
| SG-10 | 3.73 | 4.39 | 6.39 |
Electro-dialysis (ED) experiments are performed to assess the suitability of the developed membranes for the water desalination. Experiments were performed at constant applied potential (2.0 V per cell pair) using 0.1 mol dm−3 NaCl solution as feed for both of DC and CC and Na2SO4 solution (0.1 mol dm−3) through both electrode rinse compartments, in the recirculation mode. The suitability of the developed membranes for desalination applications is assessed in terms of estimating current efficiency and energy consumption for salt removal. Variation in current with time during the ED experiment for different membranes is presented in Fig. 9(A). Current at the constant applied potential decreases with time as the ions migrate from DC to CC as shown in the figure. The time versus concentration profile for DC during electrodialysis is shown in Fig. 9(B). The concentrations of DC depleted with time in a linear fashion. The concentration of DC depleted faster with the SG-10 membrane than others due to its higher ionic conductivity that facilitates the transport of ions from one compartment to the other. The concentration of DC decreases from 0.1–0.009 mol dm−3 in 118 minute with the SG-10 membrane while it takes 160 minute time with the SPES membrane for the same concentration (Fig. 9(B)). The change in the conductivity of DC and CC are shown in Fig. 9(C), as conductivity of DC decreases its increases for CC. The decrement in conductivity of DC with the SG-10 membrane is faster than with SPES due to its higher ionic conductivity. No remarkable change is recorded in pH during electrodialysis experiments.
Flux for NaCl migration from DC can be obtained by eqn (1), considering negligible mass (water) transport through membranes. Table 3 shows the NaCl flux for different composite membranes at constant potential. Ionic flux across the composite membranes followed the trend: SG-10 > SG-5 > SG-2 > SG-1 > SPES. This observation suggested that the rate of ion transport across SG-10 is higher than others. Table 3 shows the power consumption and current efficiency data to evaluate the performance of the composite membranes. It is observed that P decreased, while CE increased with the increase in GO content in the membrane matrix. Efficiency parameters (J, P and CE) depend on the operating conditions of the ED cell as well as nature and electrochemical properties of the membranes. The CE and P for the SG-10 membrane are found to be 97.4% and 4.30 kW h kg−1 respectively, while 87.1% and 5.4 kW h kg−1 for SPES membrane which shows the potential of the composite membrane for electrochemical devices.
| Membrane | Flux/mol m−2 h−1 | η | P/kW h kg−1 salt |
|---|---|---|---|
| SPES | 2.94 | 87.1 | 5.40 |
| SG-1 | 3.27 | 91.0 | 4.85 |
| SG-2 | 3.42 | 91.8 | 4.60 |
| SG-5 | 3.51 | 93.7 | 4.44 |
| SG-10 | 3.71 | 97.4 | 4.30 |
Conclusion
Graphene oxide nano sheet of 0.264 μm2 have been successfully synthesised by modified Hummer's methods. The elastic modulus value for the SG-10 composite membrane is found to be 2.9 GPa, which is 35% higher than SPES membrane; similarly 16% gain is observed in the glass transition temperature which is 240 °C for SG-10. The increase in ionic conductivity with temperature measurements revealed that GO did not affect the phase separated morphology or the ion conduction mechanism, which is also supported by thermogravimetric analysis. The high ionic conductivity of the GO/SPES nano-composite membrane is from the chemical interactions between GO/SPES, and also the presence of different oxygen functionalities on GO, which markedly enhanced ionic transport. Fig. 10 summarized the influence of GO on the different membrane properties relevant for electrochemical application. SG-10 composite membranes demonstrated the highest ionic conductivity and selectivity that is 6.4 × 10−2 S cm−1 and 6.27 × 105 cm2 S−1 respectively. The value for ionic conductivity is 2.74 times and selectivity is five times higher than that of SPES which are 2.34 × 10−2 S cm−1 and 1.28 × 105 cm2 S−1 respectively. The power consumption and current efficiency values during salt removal using the SG-10 membrane are calculated to be 4.3 kW h kg−1 and 97.4% respectively while ionic flux across the membrane is calculated to be 3.51 mol m−2 h−1. Power consumption is 20% lower and 12% higher current efficiency is achieved compared to the SPES membrane. According to the results the nano-composite membrane is highly stable and shows the highest ionic transport efficiency. Incorporation of GO in the SPES is confirmed to be an excellent scheme to enhance the transport and mechanical properties of SPES membranes.Acknowledgements
CSIR-CSMCRI communication number: 051/2014. Authors are grateful to Department of Science and Technology, New Delhi, for sponsoring of project no. DST/TM/WTI/2k10/244/A. Instrumental support received from Analytical Science Division, CSIR-CSMCRI is gratefully acknowledged.References
- K. Miyatake, T. Tombe, Y. Chikashige, H. Uchida and M. Watanabe, Angew. Chem., Int. Ed., 2007, 46, 6646 CrossRef CAS PubMed.
- H. L. Lin and S. H. Wang, J. Membr. Sci., 2014, 452, 253 CrossRef CAS PubMed.
- A. Omosebi and R. S. Besser, J. Power Sources, 2013, 228, 151 CrossRef CAS PubMed.
- S. Gahlot, P. P. Sharma, V. Kulshrestha and P. K. Jha, ACS Appl. Mater. Interfaces, 2014, 6, 5595 CAS.
- T. Chakrabarty, A. M. Rajesh, A. Jasti, A. K. Thakur, A. K. Singh, S. Prakash, V. Kulshrestha and V. K. Shahi, Desalination, 2011, 282, 2 CrossRef CAS PubMed.
- S. K. Nataraj, S. Sridhar, I. N. Shaikha, D. S. Reddy and T. M. Aminabhavi, Sep. Purif. Technol., 2007, 57, 185 CrossRef CAS PubMed.
- S. K. Nataraj, K. M. Hosamani and T. M. Aminabhavi, Desalination, 2007, 217, 181 CrossRef CAS PubMed.
- B. G. Choi, J. Hong, Y. C. Park, D. H. Jung, W. H. Hong, P. T. Hammond and H. S. Park, ACS Nano, 2011, 5, 5167 CrossRef CAS PubMed.
- S. J. Peighambardoust, S. Rowshanzamir and M. Amjadi, Int. J. Hydrogen Energy, 2010, 35, 9349 CrossRef CAS PubMed.
- C. Klaysom, R. Marschall, L. Wang, B. P. Ladewiga and G. Q. Max Lu, J. Mater. Chem., 2010, 20, 4669 RSC.
- E. Burgaz, H. Lian, R. H. Alonso, L. Estevez, A. Kelarakis and E. P. Giannelis, Polymer, 2009, 50, 2384 CrossRef CAS PubMed.
- E. Celik, H. Park, H. Choi and H. Choi, Water Res., 2011, 45, 274 CrossRef CAS PubMed.
- F. Fornasiero, H. G. Park, J. K. Holt, M. Stadermann, C. P. Grigoropoulos, A. Noy and O. Bakajin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17250 CrossRef CAS PubMed.
- K. S. Roelofs, T. Hirth and T. Schiestel, J. Membr. Sci., 2010, 346, 215 CrossRef CAS PubMed.
- B. G. Choi, H. S. Park, T. J. Park, M. H. Yang, J. S. Kim and S. Y. Jang, ACS Nano, 2010, 4(5), 2910 CrossRef CAS PubMed.
- H. Zarrin, D. Higgins, Y. Jun, Z. Chen and M. Fowler, J. Phys. Chem. C, 2011, 115, 20774 CAS.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112(11), 6027 CrossRef CAS PubMed.
- C. Y. Tseng, Y. S. Ye, M. Y. Cheng, K. Y. Kao, W. C. Shen, J. Rick, J. C. Chen and B. J. Hwang, Adv. Energy Mater., 2011, 1, 1220 CrossRef CAS.
- J. R. Potts, D. R. Dreyer, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5 CrossRef CAS PubMed.
- Y. C. Cao, C. Xu, X. Wu, X. Wang, L. Xing and K. Scott, J. Power Sources, 2011, 196, 8377 CrossRef CAS PubMed.
- B. M. Ganesh, A. M. Isloor and A. F. Ismail, Desalination, 2013, 313, 199 CrossRef CAS PubMed.
- A. K. Thakur, S. Gahlot, V. Kulshrestha and V. K. Shahi, RSC Adv., 2013, 3, 22014 RSC.
- J. Li, J. K. Park, R. B. Moore and L. A. Madsen, Nat. Mater., 2011, 10, 507 CrossRef CAS PubMed.
- D. W. Lee, V. L. D. Los Santos, J. W. Seo, L. L. Felix, A. D. Bustamante, J. M. Cole and C. H. W. Barnes, J. Phys. Chem. B, 2010, 114, 5723 CrossRef CAS PubMed.
- C. Zeng, Z. Tang, B. Guo and L. Zhang, Phys. Chem. Chem. Phys., 2012, 14, 9838 RSC.
- A. N. Mondal, B. P. Tripathi and V. K. Shahi, J. Mater. Chem., 2011, 21, 4117 RSC.
- V. Kulshrestha, G. Agarwal, K. Awasthi, B. Tripathi, N. K. Acharya, D. Vyas, V. K. Saraswat, Y. K. Vijay and I. P. Jain, Micron, 2010, 41, 390 CrossRef CAS PubMed.
- V. Kulshrestha, Int. J. Hydrogen Energy, 2009, 34, 9274 CrossRef CAS PubMed.
- R. Kumar, C. Xu and K. Scott, RSC Adv., 2012, 2, 8777 RSC.
- S. Singh, A. Jasti, M. Kumar and V. K. Shahi, Polym. Chem., 2010, 1, 1302 RSC.
- M. Kumar, S. Singh and V. K. Shahi, J. Phys. Chem. B, 2010, 114, 198 CrossRef CAS PubMed.
- H. Strathmann, Electrodialysis, in Membrane Separations Technologies Principles and Applications, ed. R. D. Noble and S. A. Stern, Elsevier Press, Ireland, 1995 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra02216e |
| This journal is © The Royal Society of Chemistry 2014 |