Nanostructures by self-assembly of polyglycidol-derivatized lipids†
Abstract
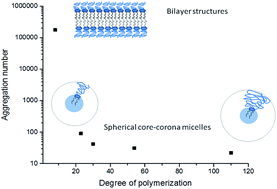
In this work we report on the self-assembly of five non-phospholipid polyglycidol conjugates in aqueous solution. The polymers are composed of a linear polyglycidol chain (degrees of polymerization, DP, are in the 8–110 range) linked to a strongly hydrophobic lipid-mimetic residue. Their behavior in dilute aqueous solution is investigated by a combination of experimental techniques – UV-vis spectroscopy, static and dynamic light scattering, fluorescence measurements, conventional and cryogenic transmission electron microscopy, and small angle X-ray scattering. The polymers spontaneously self-associate above a certain critical concentration, which depends on polyglycidol DP and temperature. According to the thermodynamic data, the self-assembly is an enthalpically disfavored endothermic process, driven by positive entropy contribution. The polymers with polyglycidol DP of 23 and above form small core–corona micelles. The latter are parameterized and the experimental values are compared to those of micelles of the commercially available poly(ethylene glycol)-derivatized lipids and other related non-phospholipid poly(ethylene glycol) conjugates. The polymer of the lowest polyglycidol DP form lamellar structures of co-existing morphology – spherical vesicles and highly anisotropic, elongated bilayer flakes.

 Please wait while we load your content...
Please wait while we load your content...