Dimethyl carbonate synthesis from carbon dioxide and methanol over CeO2 versus over ZrO2: comparison of mechanisms
Lei Chen,
Shengping Wang*,
Jingjie Zhou,
Yongli Shen,
Yujun Zhao and
Xinbin Ma
Key Laboratory for Green Chemical Technology, School of Chemical Engineering and Technology, Tianjin University, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin 300072, China. E-mail: spwang@tju.edu.cn; Fax: +86-22-87401818; Tel: +86-22-87401818
First published on 19th June 2014
Abstract
A comparison of the mechanisms of dimethyl carbonate (DMC) formation directly from carbon dioxide and methanol over CeO2 versus over ZrO2 is made through in situ Fourier transform infrared spectroscopy (FTIR). During the reaction involving methanol and CO2 adsorption over CeO2, a new band appears at around 1295 cm−1. Combining this result with in situ FTIR results of methyl formate adsorption, this band is assigned to carbomethoxide, which is taken as the intermediate in DMC formation over the ceria surface. Carbomethoxide originates from the reaction of methanol and adsorbed carbon dioxide; its formation is followed by reaction with a methoxy group to form DMC. This mechanism differs from that occurring on zirconium oxide, in which DMC is formed by the reaction between monodentate methyl carbonate and methanol.
Introduction
Carbon dioxide conversion has been attracting great interest because of concerns about global warming and sustainable development in society. There are several strategies for CO2 utilization;1−4 among these, a promising approach is direct synthesis, from CO2 and methanol, of dimethyl carbonate (DMC), which is widely used in industrial processes, for example as a fuel additive, methylation agent and solvent. Converting CO2 into environmentally friendly compounds can not only relieve ‘greenhouse’ damage, but also provide CO2 as a carbon source alternative to coal, natural gas and petroleum.Both homogeneous and heterogeneous catalysts have been applied in this reaction system.5−9 Zirconium oxide has been proven to be a useful heterogeneous catalyst, on which the mechanism of DMC formation is well studied based on acidic and basic properties.10 In recent years, cerium oxide has been applied in many fields,11,12 and also has been demonstrated to be an effective catalyst in DMC formation as a result of the acidity and basicity on the catalyst surface.8,13 However, the presently understood mechanism of DMC formation on ceria refers to that occurring on zirconium oxide, in the absence of comprehensive investigation,8,14 which is not favorable for further modifying the catalyst surface and enhancing catalytic activity. Recently, CeO2 has been attracting interest for its high oxygen storage capacity in CO2 activation, and some researchers15,16 have confirmed that CO2 can be activated on a ceria surface. It would be helpful if CO2 could be activated more easily in DMC formation. When CO2 activation is involved in the mechanism, the elementary steps should be different from that on zirconium; however, most researchers do not take this into account when they present the mechanism occurring on the ceria catalyst.8,14
In this paper, CeO2 and ZrO2 were prepared by the sol–gel method. In situ Fourier transform infrared spectroscopy (FTIR) was applied to track each reaction step to identify any differences between the mechanisms involving ceria catalyst and zirconium oxide.
Experimental design
Catalyst preparation
The CeO2 and ZrO2 were prepared by the sol–gel method with Ce(NO3)3·6H2O and Zr(NO3)4·4H2O as precursors. Solutions were made from 50 ml deionized water in which 5.211 g Ce(NO3)3·6H2O or 1.288 g Zr(NO3)4·4H2O was dissolved; then the solutions were transferred into 250 ml beakers and 5.04 or 1.26 g citric acid was added to the cerium solution or zirconium solution, respectively. The mixed solutions were stirred for 3 h under 353 K, then vaporized at the same temperature to remove the water until sols were obtained. The sols were dried at 373 K overnight and then calcinated at 873 K for 5 h.Catalytic test
Direct formation of DMC from CO2 and methanol was carried out in an autoclave. Catalyst (0.1 g) and 15 ml CH3OH were put into the autoclave. The reactor was pressurized with 5 MPa CO2. Then the reactor was heated to 413 K and stirred for 2 h. As an internal standard substance, 1-propanol was added to quantify the mixture. Products were analyzed with a gas chromatograph (GC, Agilent 4890 D).Catalyst characterization
X-ray diffraction was measured with a Rigaku D/max 2500 diffractometer equipped with Cu Kα radiation at a diffraction angle ranging from 10° to 90° with a scanning rate of 8° min−1.In situ FTIR was carried out using a Nicolet spectrometer with an MCT detector. Four scans were averaged with a resolution of 4 cm−1. Before the experiment, the samples were pressed into a disc and transferred to a homemade reaction cell that used ZnSe windows. The temperature of the cell was controlled with a programmable temperature controller.
The disc was pretreated under a He stream at 673 K for 1 h; then the sample was cooled to reaction temperature in the He stream before exposure to reactant stream. The entire process was conducted at 0.1 MPa. The flow rates of He and CO2 were 15 ml min−1 and 10 ml min−1, respectively. The He stream was passed through a drying agent to remove water, and was deoxidized in deoxidizing tube. DMC, methanol, formic acid and methyl formate were introduced into the reaction unit by the He stream through a bubbling sampler, which was maintained at 298 K.
Results
Table 1 shows the catalytic activities of CeO2 and ZrO2 for the synthesis of DMC from CO2 and methanol. DMC is the only product under the experimental conditions. It can be clearly seen that CeO2 exhibits more favorable activity in DMC synthesis than ZrO2, which is consistent with the results of other researchers.17,18| ZrO2 | CeO2 | |
|---|---|---|
| mmol DMC per g cat | 0.21 | 5.34 |
Fig. 1 shows the XRD patterns of CeO2 and ZrO2 catalysts. As shown in Fig. 1(a), diffraction peaks at 2θ = 28.6°, 33.1°, 47.6° and 56.4° are associated with the cubic fluorite phase of CeO2, corresponding to the planes of (111), (200), (220) and (311), respectively. In Fig. 1(b), diffractions shown at 2θ = 28.2° and 31.5° are assigned to the monoclinic phase of ZrO2, and the peak at 2θ = 30.2° is attributed to the tetragonal phase of ZrO2. This implies that ZrO2 is a mix of monoclinic and tetragonal phases, which accords with the result of Parghi.19 There are no other diffraction peaks in ZrO2 or CeO2, indicating that ZrO2 and CeO2 are both at high purity.
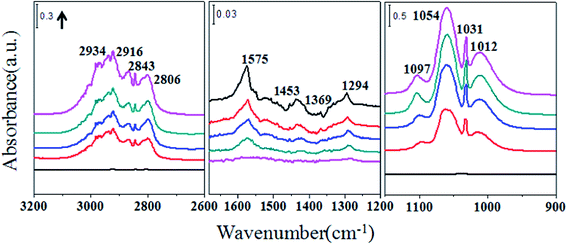
Fig. 2 shows the IR spectra of methanol adsorption on ceria catalysts. Methanol interacts with the surface of ceria catalyst via the oxygen atoms. Features for ν(CO) of methoxy grow simultaneously at 1097, 1054 and 1012 cm−1, attributed to on-top methoxy, bridging methoxy and three coordinate methoxy groups, respectively.20 The intensity of the on-top methoxy band rises more slowly than those of the others. The band around 1031 cm−1 may be ascribed to bridging methoxy or physisorbed methoxy. In the ν(CH3) region there are two types of vibration: bands at 2806 and 2915 cm−1 are associated with the modes of ν(CH3) and νas(CH3) of chemisorbed methoxy; 2834 and 2934 cm−1 are attributed to symmetric and asymmetric CH3 stretching modes of physisorbed methanol. Bands observed at 1575, 1453 and 1369 cm−1 represent monodentate methyl carbonate (MMC). A band also emerges around 1295 cm−1, which so far has not been assigned. Fig. 3 shows the spectra of successive methanol adsorption on ZrO2 surface, which is identical with the results of other researchers,21 except for the intensity. Bands at 1162 and 1032 cm−1 are associated with the bending vibration of methoxy groups, and bands at 2822 and 2924 cm−1 with increasing intensity are ascribed to the C–H stretching vibration of bidentate and monodentate methoxy groups. The decreasing absorbance of bands (3768, 3686 and 3674 cm−1) is associated with different types of OH groups on the ZrO2 surface.22
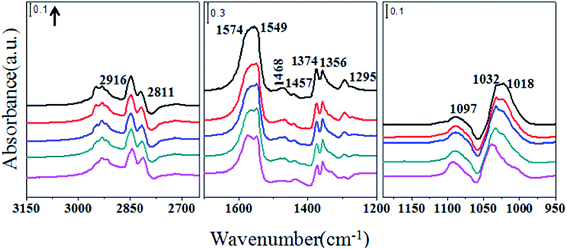
Exposure of ceria catalysts containing preadsorbed methanol to the CO2 stream changes the infrared spectra remarkably. As seen in Fig. 4, the band representing on-top methoxy decreases, but the features for MMC (1574, 1457 and 1374 cm−1) increase. The band at 1295 cm−1 also increases, although it does not have a specific assignment. Bands at 1468 and 1356 cm−1 are associated with monodentate carbonate. Peaks at 1549 and 1018 cm−1 indicate the appearance of bidentate carbonate.23,24 Infrared spectra taken during exposure of ZrO2 containing preadsorbed methanol to CO2 are shown in Fig. 5. Bands for on-top and bridging methoxy (1158 and 1032 cm−1) decrease and new peaks appear at 1596, 1497, 1471, 1391, 1362, 1200 and 1110 cm−1, which are ascribed to MMC.10 The formation of MMC can also be confirmed through the C–H stretching vibration. Bands (2924 and 2818 cm−1) for methoxy species decrease, and new bands at 2960 and 2880 cm−1 appear.10 The peak around 1295 cm−1 does not appear during this process.
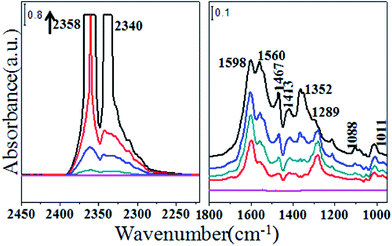
Fig. 6 shows the infrared spectra recorded on adsorption of CO2 on CeO2. Bands for νas(CO2) in gas phase are clearly observed at 2358 and 2340 cm−1. Bands at 1560, 1289 and 1011 cm−1 stem from ν(CO3) of bidentate carbonate,23,24 whereas 1467, 1352 and 1088 cm−1 are features for ν(CO3) of monodentate carbonate.23,25 Peaks at 1598 and 1413 cm−1 are ascribed to hydrogen carbonate.23,26 Spectra of ZrO2 reacting with CO2 at 413 K are shown in Fig. 7. The clear bands at 1221, 1425, 1626 and 1683 cm−1 are assigned to bicarbonate, and 1366 cm−1 may indicate the presence of bidentate carbonate.27
Fig. 8 displays the spectra of methanol successive introduction to CeO2 after CO2 adsorption. Peaks appear in ν(CH3) and ν(CO) regions representing all different kinds of methoxy. New weak bands at 1572, 1454 and 1369 cm−1 are assigned to MMC. The unassigned band at 1295 cm−1 arises, too. Features (1602 and 1410 cm−1) for hydrogen carbonate decrease in the methanol stream. The intensity of bands (1463 and 1354 cm−1) for monodentate carbonate reduces, too. Fig. 9 illustrates the transformation of spectra when methanol starts to adsorb on ZrO2 containing preadsorbed CO2. New bands appearing at 1165 and 1033 cm−1 are for top- and bridging-methoxy, respectively. Features for 1605, 1463 and 1354 cm−1 are associated with MMC.28 There is no sign of a band around 1295 cm−1 during the CO2 adsorption process.
Fig. 10 shows the infrared spectra of DMC adsorbed on ceria catalysts. In the ν(CH3) region, bands at 2910 and 2804 cm−1, which are assigned to νas(CH3) and νs(CH3) of methoxy, respectively, increase in intensity with time. Bands at 2965 and 2869 cm−1 are associated with the νas(CH3) and νs(CH3) of DMC. Bands at 1780 and 1767 cm−1 may relate to the physisorbed DMC because these bands can be totally removed when the sample is exposed to the pure He stream. Features at 1109 and 1061 cm−1 are associated with the ν(CO) of on-top methoxy and bridging methoxy. The unassigned peak around 1295 cm−1 appears, too, with a great increase. Fig. 11 records the infrared spectra of DMC passing over ZrO2. Bands at 1603, 1357 and 1200 cm−1 indicate the appearance of MMC on zirconium.28 The on-top methoxy is evident with the rising band at 1166 cm−1. Other types of methoxy also appear during the DMC adsorption process.
Fig. 12 illustrates the transformation of infrared spectra when CeO2 is exposed to HCOOH in the He stream. The bands are ascribed to formate species,23 i.e. ν(C–H), 2842 cm−1, νas(OCO), 1590, 1557, δ(C–H), 1371 cm−1, νs(OCO), 1353, 1252 cm−1. The peak at 2925 cm−1 results from the combination band of νas(OCO) and δ(C–H).29 It has been indicated that the frequency differences between νas(OCO) with νs(OCO) retain the following sequence: monodentate formate > free formate ion > bidentate formate. According to this empirical approach, bands at 1590 and 1252 cm−1 may result from monodentate formate because the frequency separation is 338 cm−1, which is much larger than that of free formate ion (250 cm−1). The remaining features (1557, 1353 cm−1) are associated with bidentate formate as the frequency separation is 204 cm−1, less than 250 cm−1. During the adsorption process, there is no sign of the peak at around 1295 cm−1.
The spectra of methyl formate adsorption on ceria catalysts evolve in a complicated way. Bands observed at 3040, 3008, 2967, 2947, 1766 and 1753 cm−1 in Fig. 13 are assigned to methyl formate physisorbed on cerium oxide.30 The peak at 2926 cm−1 is associated with the combination band of νas(OCO) and δ(C–H). Bands at 2844, 1599, 1559, 1377, 1353 and 1248 cm−1 are from the formate adsorbed on the surface. Shoulders at 2915 and 2801 cm−1 are similar to those peaks that result from the exposure of ceria catalysts to methanol. In the region of ν(CO) bands at 1032 and 1020 cm−1 are associated with bridging and tri-coordinate methoxy. It is worth mentioning that, as expected, the band at around 1295 cm−1 appears.
Discussion
The spectra of methanol adsorption on ceria and zirconium10,20,31 are shown in Fig. 2 and 3. It is indicated that CH3OH adsorbs on these two catalysts through O atoms. The H atom of hydroxyl in methanol can react with OH on the metal oxide surface, or a coordinately unsaturated O2− on the surface, to produce hydroxyl. On the ZrO2 surface, the negative features of ν(OH) in Fig. 3 indicate that the H atom combines with the OH to produce H2O. On the ceria surface, however, the H atom associated with hydroxyl of methanol reacts with the coordinately unsaturated O2−, resulting in formation of an OH group, as can be observed in Fig. 2. This result is also confirmed by those of other researchers.31 The big differences between methanol adsorption on pure ceria and zirconium oxide are the appearance of MMC and the band at around 1295 cm−1. Peaks of MMC and 1295 cm−1 appear only when CH3OH adsorbs on ceria catalysts, which cannot be detected on ZrO2 surface. Based on this result, it is proposed that MMC formation on ceria surface comes from the reaction between methanol and adsorbed CO2, which is seldom mentioned by other researchers.When CO2 is introduced to ceria catalysts containing preadsorbed methanol, the intensity of on-top methoxy, which is also considered to be the reactive methoxy by other researchers,8,32 decreases because of the reaction with CO2. The spectra of CO2 introduction onto zirconium oxide surface after methanol adsorption are similar to those of earlier reports,33 and agree with the observation that on-top methoxy is more reactive than other types.10 Noticeably, bands representing MMC are observed with both catalysts during this CO2 adsorption process, whereas the band at around 1295 cm−1 is detected only with CeO2. It can be deduced that this band (1295 cm−1) on the ceria surface does not originate from single CO2 adsorption, as shown in Fig. 6. By comparison, it is noted that the band at 1295 cm−1 arises again when the ceria catalyst is exposed to successive introduction of methanol after CO2 adsorption, further confirming that this band (1295 cm−1) results from the reaction of methanol with adsorbed CO2.
As can be seen in Fig. 8, features for hydrogen carbonate and monodentate carbonate all decrease during methanol adsorption on CeO2 containing preadsorbed CO2, indicating that those kinds of adsorbed CO2 may be involved in the reaction with methoxy. These structures lead to CO2 activation. The specific reactive structure of CO2 is not detailed in this paper because the activated structures depend on the type of oxygen vacancies, crystal face and coverage of CO2. It is unreliable to propose a specific structure merely from the results of infrared spectra. The corresponding DFT calculation is in progress.
Further investigation was conducted to decide whether this band (1295 cm−1) represents an intermediate or not. It is a common phenomenon that features for DMC are difficult to observe under operating experimental conditions because of its low concentration. However, according to the theory of microreversibility, decomposition of a compound should go through the same elementary steps as formation of that compound. Based on this concept, decomposition of DMC would reveal the intermediate needed to produce DMC. The decomposition of DMC on ZrO2 surface is shown in Fig. 11. Features for MMC and methoxy appear in the adsorption process without the band at around 1295 cm−1. Hence, MMC is the intermediate in DMC formation over the zirconium surface. When CeO2 is exposed to DMC, the band at about 1295 cm−1 and features for carbonates appear, as observed in Fig. 10. During this process, peaks for MMC are invisible even after long time exposure, which is very different from the results seen on ZrO2. As mentioned above, instead of the single adsorption, a band near 1295 cm−1 originates from the reaction between methoxy and CO2. Depending on the result of DMC adsorption and the theory of microreversibility, it is concluded that the peak at 1295 cm−1 must be an important feature indicative of the intermediate in DMC formation from CO2 and methanol on the CeO2 surface, meaning that there is a different mechanism in DMC formation on ceria catalysts.
So far, the band at about 1295 cm−1 has not been assigned, but some helpful hints contribute to definition of this band. Li et al.29 observed a band near 1300 cm−1 and associated this band with the νs(OCO). Combining the characteristic of this reaction with the band representing νs(OCO), we propose that this band may be a feature of carbomethoxide (CH3OCO). To test this assumption, methyl formate adsorption was conducted on the CeO2 surface. It is noted that a weak band appears at around 1295 cm−1 (1291 cm−1), as observed in Fig. 13. It is probable that appearance of the band at about 1295 cm−1 comes from methoxy and formate because esters easily decompose into alcohols and carboxylic acids. So, HCOOH adsorption on CeO2 was undertaken to verify whether the band at about 1295 cm−1 stems from formic acid adsorption or not. As exhibited in Fig. 12, bands for νs(OCO) appearing at 1353 and 1248 cm−1 are far from 1295 cm−1, indicating that it is hard to link the band at about 1295 cm−1 with formic acid adsorption. Moreover, HCOOH formation from methanol on the surface of ceria is unfavorable under the experimental conditions,34 further demonstrating that this band does not come from formic acid adsorption. During the methyl formate adsorption process, it is also noted that there are no features for on-top methoxy, which is well accepted as the reactive methoxy in carbomethoxide formation. Hence, the rising band at around 1295 cm−1 in the methyl formate adsorption process does not result from reaction between methoxy and CO2. Appearance of the band at around 1295 cm−1 can be explained as follows. The C–H bond that the corresponding C atom links with two oxygen atoms may break up to form an H atom and –OCOCH3. The H atom can react with the OH on CeO2 surface, and the remaining part (–OCOCH3) may attach to the ceria atom on the surface, resulting in emergence of a peak at around 1295 cm−1. Although methyl formate adsorption on ceria surface is not direct evidence of assignment of the new band at 1295 cm−1 in DMC formation, it can explain this result to some extent.
Thus, the mechanism of DMC formation directly from CO2 and methanol over a CeO2 catalyst is described as below, which is different from that previously suggested by other researchers.8,14 In this mechanism, CO2 adsorbs on the CeO2 surface to produce adsorbed CO2 noted as CO2–CeO2, as shown in Reaction (1). Reaction (2) involves adsorption of methanol on the ceria surface. Carbomethoxide forms through interaction between the adsorbed CO2 and methanol, as depicted in Reaction (3). Methoxy will react with the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in CO2 to form carbomethoxide. The remaining oxygen atom of CO2 combines with the hydrogen from the hydroxyl in methanol to produce hydroxyl on the surface of CeO2. Then the carbomethoxide reacts with another methanol to produce DMC, which can be seen in Reaction (4). The formation of carbomethoxide is also strongly supported by the decomposition of DMC.
O in CO2 to form carbomethoxide. The remaining oxygen atom of CO2 combines with the hydrogen from the hydroxyl in methanol to produce hydroxyl on the surface of CeO2. Then the carbomethoxide reacts with another methanol to produce DMC, which can be seen in Reaction (4). The formation of carbomethoxide is also strongly supported by the decomposition of DMC.
| CO2 + CeO2 → CO2–CeO2 | (1) |
| CH3OH + CeO2 → CH3OH–CeO2 | (2) |
| CH3OH–CeO2 + CO2–CeO2 → CH3OCO–Ce + OH–Ce | (3) |
| CH3OCO–Ce + OH–Ce + CH3OH → (CH3O)2CO + H2O + CeO2 | (4) |
Conclusions
This study investigated the mechanism of DMC formation directly from methanol and CO2 catalyzed by CeO2 compared with that obtained over ZrO2. Based on the results of in situ FTIR, DMC formation from CO2 and methanol over a ceria catalyst is proposed to proceed via a new and different mechanism from that on ZrO2. On a ZrO2 surface, DMC is formed by the reaction of monodentate methyl carbonate with methanol. In contrast, on a ceria surface, methanol reacts with the adsorbed CO2 to produce a new intermediate, carbomethoxide. A feature of carbomethoxide is observed at around 1295 cm−1, which is defined through adsorption of methyl formate. Then DMC is formed via the reaction between carbomethoxide and methoxy produced from the dissociation of methanol. DMC decomposition is observed to proceed via the reverse of this route, further confirming the formation of DMC directly from CO2 and methanol through a carbomethoxide intermediate.Acknowledgements
The authors gratefully acknowledge financial support by the Natural Science Foundation of China (NSFC) (Grant no. 21176179), the Program for New Century Excellent Talents in University (NCET-13-0411), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (MoE) and the Program of Introducing Talents of Discipline to Universities (B06006).References
- M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2013, 113, 1709–1742 Search PubMed.
- W. Wang, S. Wang, X. Ma and J. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- Y. Izumi, Coord. Chem. Rev., 2013, 257, 171–186 CrossRef CAS PubMed.
- N. A. M. Razali, K. T. Lee, S. Bhatia and A. R. Mohamed, Renewable Sustainable Energy Rev., 2012, 16, 4951–4964 CrossRef CAS PubMed.
- J.-C. Choi, T. Sakakura and T. Sako, J. Am. Chem. Soc., 1999, 121, 3793–3794 CrossRef CAS.
- A. Dibenedetto, C. Pastore and M. Aresta, Catal. Today, 2006, 115, 88–94 CrossRef CAS PubMed.
- K. Tomishige, T. Sakaihori, Y. Ikeda and K. Fujimoto, Catal. Lett., 1999, 58, 225–229 CrossRef CAS.
- Y. Yoshida, Y. Arai, S. Kado, K. Kunimori and K. Tomishige, Catal. Today, 2006, 115, 95–101 CrossRef CAS PubMed.
- J. Bian, M. Xiao, S. Wang, Y. Lu and Y. Meng, Catal. Commun., 2009, 10, 1142–1145 CrossRef CAS PubMed.
- K. T. Jung and A. T. Bell, J. Catal., 2001, 204, 339–347 CrossRef CAS.
- C. Sun, H. Li and L. Chen, Energy Environ. Sci., 2012, 5, 8475–8505 CAS.
- M. B. Gawande, V. D. B. Bonifacio, R. S. Varma, I. D. Nogueira, N. Bundaleski, C. A. A. Ghumman, O. M. N. D. Teodoro and P. S. Branco, Green Chem., 2013, 15, 1226–1231 RSC.
- S. Wang, L. Zhao, W. Wang, Y. Zhao, G. Zhang, X. Ma and J. Gong, Nanoscale, 2013, 5, 5582–5588 RSC.
- H. J. Hofmann, A. Brandner and P. Claus, Chem. Eng. Technol., 2012, 35, 2140–2146 CrossRef CAS.
- S. Bernal, G. Blanco, J. Gatica, C. Larese and H. Vidal, J. Catal., 2001, 200, 411–415 CrossRef CAS.
- Z. Cheng, B. J. Sherman and C. S. Lo, J. Chem. Phys., 2013, 138, 014702 CrossRef PubMed.
- K. Tomishige and K. Kunimori, Appl. Catal., A, 2002, 237, 103–109 CAS.
- H. Lee, S. Park, I. Song and J. Jung, Catal. Lett., 2011, 141, 1–7 CrossRef PubMed.
- K. D. Parghi, S. R. Kale, S. S. Kahandal, M. B. Gawande and R. V. Jayaram, Catal. Sci. Technol., 2013, 3, 1308–1313 CAS.
- S. Rousseau, O. Marie, P. Bazin, M. Daturi, S. Verdier and V. Harlé, J. Am. Chem. Soc., 2010, 132, 10832–10841 CrossRef CAS PubMed.
- C. Binet and M. Daturi, Catal. Today, 2001, 70, 155–167 CrossRef CAS.
- D. G. Rethwisch and J. Dumesic, Langmuir, 1986, 2, 73–79 CrossRef CAS.
- G. N. Vayssilov, M. Mihaylov, P. S. Petkov, K. I. Hadjiivanov and K. M. Neyman, J. Phys. Chem. C, 2011, 115, 23435–23454 CAS.
- C. Li, Y. Sakata, T. Arai, K. Domen, K.-i. Maruya and T. Onishi, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 929–943 RSC.
- O. Pozdnyakova, D. Teschner, A. Wootsch, J. Kröhnert, B. Steinhauer, H. Sauer, L. Toth, F. Jentoft, A. Knop-Gericke and Z. Paál, J. Catal., 2006, 237, 17–28 CrossRef CAS PubMed.
- C. Binet, M. Daturi and J. C. Lavalley, Catal. Today, 1999, 50, 207–225 CrossRef CAS.
- W. Hertl, Langmuir, 1989, 5, 96–100 CrossRef CAS.
- K. T. Jung and A. T. Bell, Top. Catal., 2002, 20, 97–105 CrossRef CAS.
- C. Li, K. Domen, K.-i. Maruya and T. Onishi, J. Catal., 1990, 125, 445–455 CrossRef CAS.
- G. J. Millar, C. H. Rochester and K. C. Waugh, J. Chem. Soc., Faraday Trans., 1991, 87, 2785–2793 RSC.
- M. M. Natile, G. Boccaletti and A. Glisenti, Chem. Mater., 2005, 17, 6272–6286 CrossRef CAS.
- M. Aresta, A. Dibenedetto, C. Pastore, A. Angelini, B. Aresta and I. Pápai, J. Catal., 2010, 269, 44–52 CrossRef CAS PubMed.
- K. Tomishige, Y. Ikeda, T. Sakaihori and K. Fujimoto, J. Catal., 2000, 192, 355–362 CrossRef CAS.
- Z. Wu, M. Li, D. R. Mullins and S. H. Overbury, ACS Catal., 2012, 2, 2224–2234 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |