Synthesis, characterization and gelation studies of a novel class of rhodamine based N-glycosylamines†
Abstract
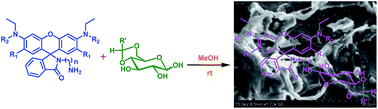
Twelve different rhodamine based N-glycosylamines were synthesized from the corresponding rhodamine based amine derivatives and 4,6-O-protected-D-glucose. All these compounds were characterized by using different spectral techniques and by their physico-chemical properties.

 Please wait while we load your content...
Please wait while we load your content...