Dimethyl carbonate synthesis from carbon dioxide and methanol over CeO2versus over ZrO2: comparison of mechanisms
Abstract
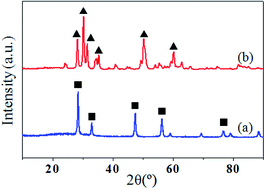
A comparison of the mechanisms of dimethyl carbonate (DMC) formation directly from carbon dioxide and methanol over CeO2 versus over ZrO2 is made through in situ Fourier transform infrared spectroscopy (FTIR). During the reaction involving methanol and CO2 adsorption over CeO2, a new band appears at around 1295 cm−1. Combining this result with in situ FTIR results of methyl formate adsorption, this band is assigned to carbomethoxide, which is taken as the intermediate in DMC formation over the ceria surface. Carbomethoxide originates from the reaction of methanol and adsorbed carbon dioxide; its formation is followed by reaction with a methoxy group to form DMC. This mechanism differs from that occurring on zirconium oxide, in which DMC is formed by the reaction between monodentate methyl carbonate and methanol.

 Please wait while we load your content...
Please wait while we load your content...